
FOI 24/25-0151

FOI 24/25-0151
FOI 24/25-0151
FOI 24/25-0151

FOI 24/25-0151

FOI 24/25-0151

FOI 24/25-0151

FOI 24/25-0151

FOI 24/25-0151
Research Request – Magnetic EEG/EKG Guided-Resonance
Therapy (MeRT)
• Please provide a summary rating the quality of evidence cited and
provided by applicant
Brief
• Please provide any further research evidence of the use of MeRT as an
intervention for a child (10 years) with ASD.
• Is MeRT considered a clinical intervention and therefore not
appropriately funded through the NDIS
Date
11/11/20
Requester
s47F - personal privacy (Senior Technical Advisor TAB)
Researcher
s47F - personal privacy (Research Team Leader)
Contents
Summary ................................................................................................................................................. 2
Magnetic EEG/EKG Guided-Resonance Therapy (MeRT)........................................................................ 2
Transcranial Magnetic Stimulation ......................................................................................................... 2
Is TMS a clinical intervention? ................................................................................................................ 3
Clinical settings for TMS .................................................................................................................. 3
Cost ................................................................................................................................................. 3
Scientific Evidence provided by the Brain Treatment Centre ................................................................. 3
Reference List .......................................................................................................................................... 9
Please note:
The research and literature reviews col ated by our TAB Research Team are not to be shared
external to the Branch. These are for internal TAB use only and are intended to assist our advisors
with their reasonable and necessary decision making.
Delegates have access to a wide variety of comprehensive guidance material. If Delegates require
further information on access or planning matters they are to call the TAPS line for advice.
The Research Team are unable to ensure that the information listed below provides an accurate &
up-to-date snapshot of these matters
Magnetic EEG/EKG Guided-Resonance Therapy (MeRT)
P a g e |
1

FOI 24/25-0151
Summary
• The evidence provided by the participant is generally of low quality
o Mainly consists of evidence for the use of MeRT for those with a diagnosis of PTSD
• No peer reviewed literature could be sourced on the use or efficacy of MeRT in people with
an ASD diagnosis
• There is some early evidence in favour of transcranial magnetic stimulation (TMS) (MeRT is a
variant of TMS) for ASD, however, published literature is of low quality and must be
regarded as preliminary and insufficient to support offering TMS to treat ASD.
• MeRT/TMS are clinical interventions which must be administered by a trained and
accredited medical/health professional. It is not covered by Medicare or Private Health
Insurance
Magnetic EEG/EKG Guided-Resonance Therapy (MeRT)
Magnetic EEG guided Resonant Treatment or Magnetic e-Resonance Therapy (MeRT) is a variation of
Transcranial Magnetic Stimulation (TMS) where personalized treatment frequencies and output
intensities are derived from patient’s EEG data and resting heart rate.
Transcranial Magnetic Stimulation
Refer to NED20/281579 for an overview of repetitive TMS which is approved for use in Australia and
recommended by the Royal Australian and New Zealand College of Psychiatrists (RANZCP) as
treatment for treatment resistant major depressive disorders.
TMS has been around for more than two decades and has data confirming its low risk profile, and
excellent tolerability. While adult trials show promise in using TMS as a novel, non-invasive, non-
pharmacologic diagnostic and therapeutic tool in a variety of nervous system disorders, its use in
children is only just emerging.
Multiple systematic reviews investigating the use of TMS’ in children and adolescents with ASD have
been published [1-4]. All reviews concluded that:
1) Treatment led to improvements in relation to repetitive behaviours, stereotypes behaviours,
social behaviours and executive function tasks.
2) Long term gains/stability were not well reported
3) Studies are of low methodological quality (case studies, non-randomised trials), included
cohorts with significant heterogeneity and lacked any control of confounding factors
Therefore, there is urgent need for randomised controlled trials of high quality with adequate follow
up periods to test the efficacy of TMS for ASD. Currently available evidence must be regarded as
preliminary and insufficient to support offering TMS to treat ASD.
Magnetic EEG/EKG Guided-Resonance Therapy (MeRT)
P a g e |
2

FOI 24/25-0151
Is TMS a clinical intervention?
TMS is a clinical intervention and should only be administered by a professional who has undertaken
training and is credentialed in the procedure. This is commonly a psychiatrist, however, psychiatry
trainees and psychiatric nurses can perform the treatment under supervision.
In Australian clinical practice TMS should only be administered for an illness where there is adequate
evidence of clinical indication and effectiveness. This includes depression, schizophrenia and
obsessive compulsive disorder. It should be considered as a therapeutic option alongside other
treatments after detailed psychiatric assessment.
Clinical settings for TMS
• TMS treatment can be conducted safely as an outpatient procedure and is predominantly
provided in this context internationally.
• TMS treatment does not require sedation or general anaesthesia.
• All services providing TMS should have in place appropriate protocols, training and
equipment to allow for the safe and effective administration of treatment. This should
include protocols for patient assessment, monitoring during treatment, monitoring of the
quality of the provision of treatment, protocols for response to adverse events and
monitoring of outcomes
• Where TMS is conducted as an outpatient the outpatient TMS clinic should be suitably
accredited by an accepted accreditation agency such as International Standards Organisation
(ISO) or Australian Council of Healthcare Standards (ACHS)
• Devices used for TMS should be approved by the Therapeutic Goods Administration (TGA)
for use in Australia or the New Zealand Medicines and Medical Devices Safety Authority for
use in New Zealand. A service using a specific TMS device should check the intended use that
has been formal y approved by these organisations, as these can differ between devices.
Cost
In Australia, TMS is not covered by Medicare or Private Health Insurance.
Scientific Evidence provided by the Brain Treatment Centre
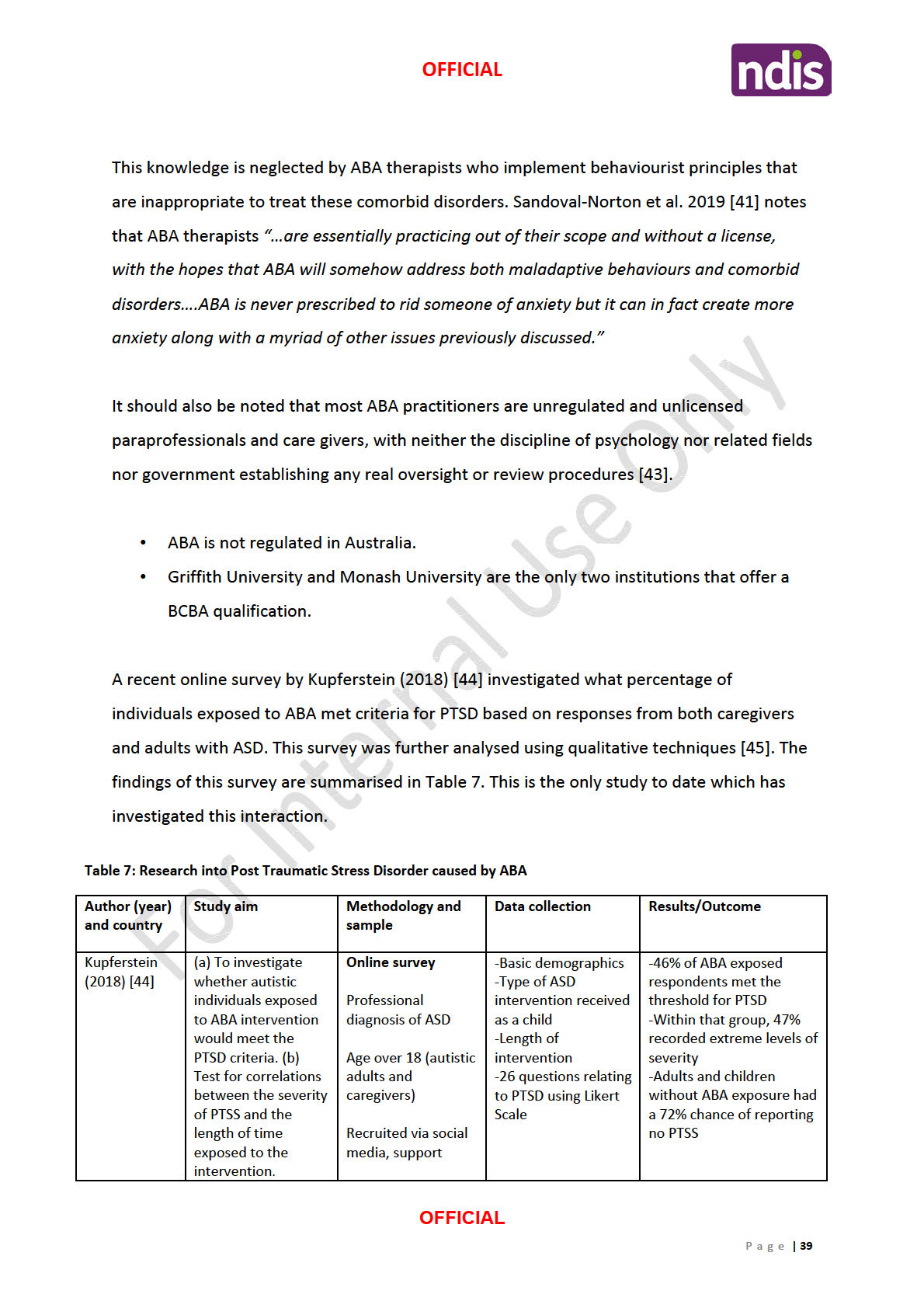
The evidence provided by the Brain Treatment Centre (Table 1) consists of:
1) Narrative review which summarises the results of systematic reviews investigating the
efficacy of TMS for major depressive disorder. This paper is of
moderate quality and shows
that TMS is a useful tool to treat
major depressive disorders but provides no scientific
information of MeRT.
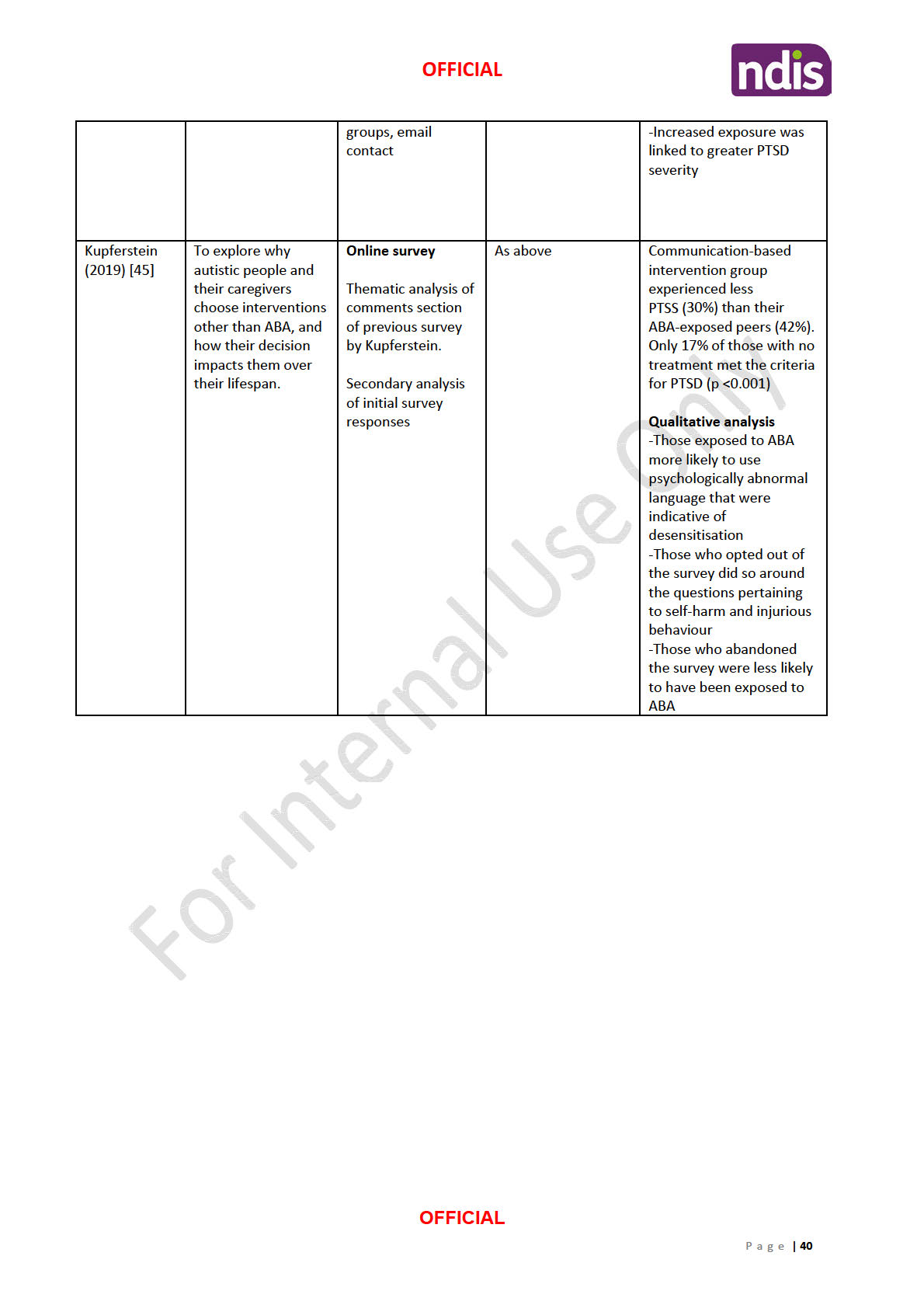
2) Cross sectional study which shows that peak alpha frequency (PAF) measures from EEG
correlates with non-verbal cognitive function in children with ASD. This measure is being
claimed to have the potential to act as a biomarker in the future, to help study whether an
autism treatment is effective in restoring peak alpha frequency to normal levels. This paper
is of
medium quality, however, provides little value in the argument for MeRT. All it shows is
Magnetic EEG/EKG Guided-Resonance Therapy (MeRT)
P a g e |
3

FOI 24/25-0151
a difference in brain waves/oscillations between normally developing children and those
with ASD, not whether MeRT is appropriate or useful as a treatment for ASD.
3) A conference presentation is the only evidence provided for the use of MeRT in children
with ASD. Although results show improvements in autism behaviours the quality of the
evidence is rated as
very low as it is not peer reviewed, retrospective data col ection and
there were high dropout rates. This information should not be used as evidence for the
effectiveness of the treatment.
4) The two remaining papers include a retrospective chart review
(low quality) and an
unpublished randomised control ed trial
(very low quality). These two papers investigate
the use of MeRT in
veterans with PTSD. Both show positive results but must be assessed
with caution due to their low methodological quality, lack of peer review and potential for
bias as the studies were not performed by an independent research group (co-creators of
MeRT conducted studies).
Magnetic EEG/EKG Guided-Resonance Therapy (MeRT)
P a g e |
4
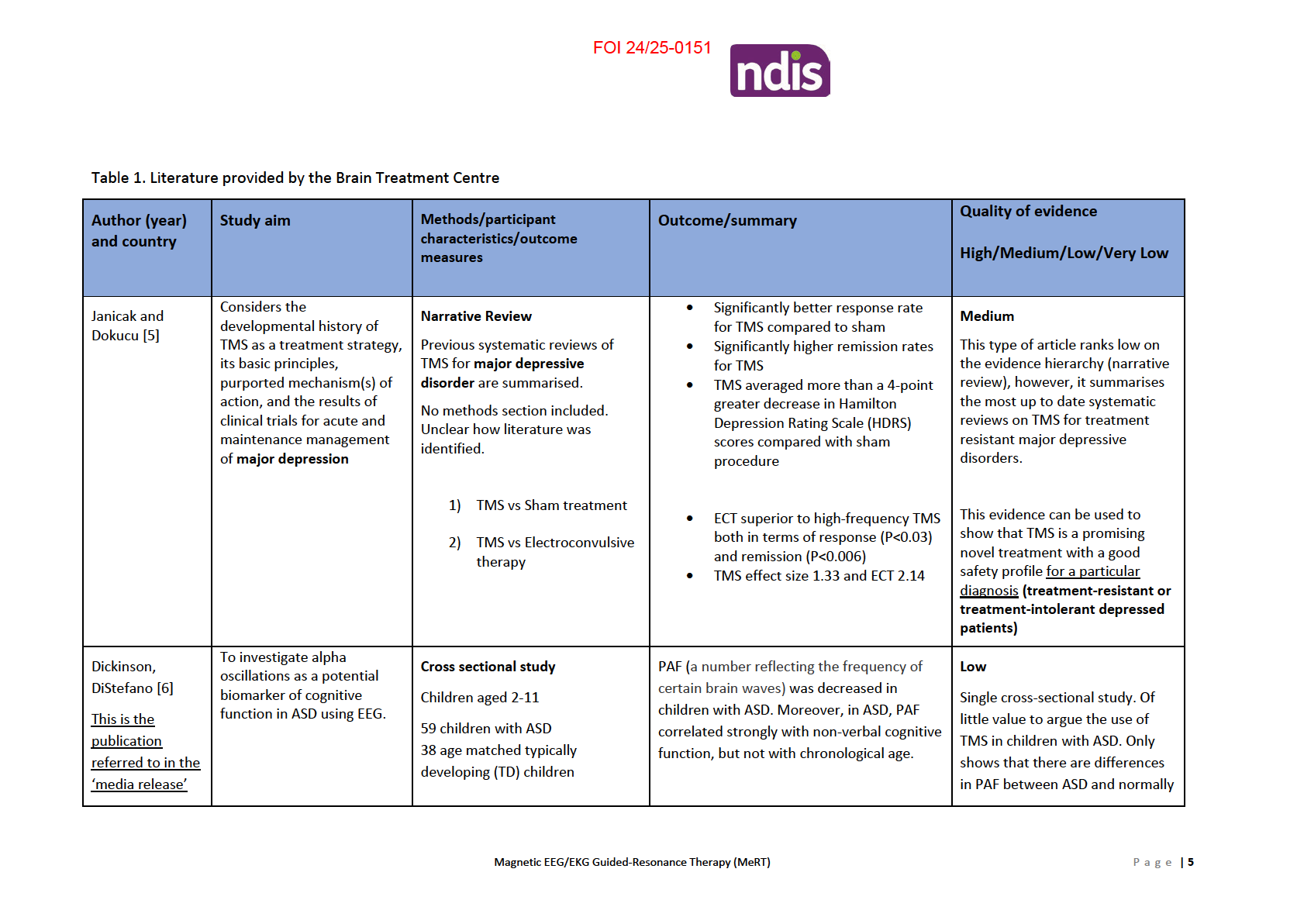

FOI 24/25-0151
provided by the
PAF may be used as a biomarker in the
developing aged matched
Brain Treatment
future, to help study whether an autism
Exclusion criteria included other
children.
Centre
treatment is effective in restoring peak alpha
neurological abnormalities
frequency to normal levels, for instance.
Furthermore, the study didn’t
(including active epilepsy), birth-
measure ASD symptoms so
related complications and
cannot infer how this may change
uncorrected vision or hearing
across severity levels.
impairment.
• Cognitive and language
assessments
• electroencephalography
(EEG) recording to
measure peak alpha
frequency (PAF)
Kim and Taghva
Hypothesize behavioural
At 24 month fol ow up, 26 of 44 (59%)
improvements in autism
Conference presentation
patients showed statistical y-significant
Very Low
[7]
behaviours via TMS with
Retrospective chart review
improvements of -11.7 +/- 6.2 S.D. Ten
Non peer reviewed, retrospective,
customized frequency
patients’ CARS fel below 26 (38%) consistent high dropout rates.
modulation.
141 patients underwent TMS with with minimization of autism behaviours.
customized frequency modulation.
This literature should not be used
Serial EEGs were used to modify
Most improvements were made in Taste,
to make a clinical treatment
frequency delivered using resting
Smel , and Touch Response and Use, Fear
alpha frequency combined with
decision.
and Nervousness, and Verbal
resting heart rate.
Communication
35 patients were excluded at 1
week due to lack of improvement
on Child Autism Score (CARS).
1 patient was excluded at first
week for seizure (0.7%).
44 (41.5%) made it to the 24-
month fol ow up period.
Magnetic EEG/EKG Guided-Resonance Therapy (MeRT)
P a g e |
6

FOI 24/25-0151
Age, sex, race, other treatments,
number of treatments, CARS
scores were sub-stratified.
Taghva, Silvetz
To determine if magnetic
Retrospective Chart Review
Of the 21 patients who initiated therapy, 16
Low
[8]
brain stimulation can induce 21 veterans consecutively-treated (76%) completed treatment. Clinical
Smal sample, no control group,
normalization of EEG
for
PTSD.
improvements on the PCL-M were seen in
lack of long term fol ow up data.
abnormalities and improve
these 16 patients, with an average pre-
clinical symptoms in
PTSD in Magnetic resonance therapy
treatment score of 54.9 and post-treatment
The study suggests that non-
a preliminary, open-label
(MRT) was administered for two
score of 31.8 (P < 0.001). In addition, relative invasive neuro-modulation
evaluation
weeks at treatment frequencies
global EEG alpha band (8 - 13 Hz) power
magnetic resonance therapy may
based on frequency-domain
increased from 32.0 to 38.5 percent (P =
lead to clinical improvements,
analysis of each patient’s
0.013), and EEG delta-band (1 - 4 Hz) power
however, further large scale, high
dominant alpha-band EEG
decreased from 32.3 percent to 26.8 percent quality studies are required
frequencies and resting heart rate. (P = 0.028)
Patients were evaluated on the
PTSD checklist (PCL-M) and pre-
and post-treatment EEGs before
and after MRT.
Taghva, Jin [9]
To determine if MeRT can
Characteristics for the randomized groups
improve clinical symptoms in
Randomised Control ed Trial
were similar, with pre-treatment average
Very Low
PTSD in a double-blind,
86 veterans (ages 20-56, mean
PCL-M scores of 65.7 and 65.4 in the MeRT
Unpublished/non-peer reviewed,
sham control ed,
37.8; 9 female, 77 male) with prior and sham stimulation groups respectively.
no power or sample size
randomized trial.
diagnosis of
PTSD (moderate to
calculations, no information on
severe, PCL-M> 50) were
74 completed. 12 (14%) dropped out of
how participants were
randomized to receive MeRT
study
versus sham stimulation for two
recruited/selected (selection bias)
weeks, fol owed by open-label
Two-week post-treatment PCL-M in the
active treatment of both groups
control arm was
for two weeks.
51.4 (28.9% improvement), and in the
treatment arm was 42.6
MeRT was administered with pulse (47.4% improvement, F1,71 = 7.4, P < 0.01)
intensity at 80% of patient motor
Magnetic EEG/EKG Guided-Resonance Therapy (MeRT)
P a g e |
7

FOI 24/25-0151
threshold and stimulation
No adverse events (seizures, neurologic
frequency based on analysis of
deficit, worsening of pre-treatment
each patient’s EEG and resting
condition) were reported.
heart rhythm.
Patients were evaluated on the
PTSD Check List – Military (PCL-M),
at pre-treatment, weeks 2 and 4 of
treatment, and three-month
fol ow up (eight weeks post
treatment).
Exclusion criteria included history
of seizure disorder, history of
intracranial lesion, and history of
intracranial implant, prior
transcranial magnetic therapy, and
inability to adhere to the
treatment schedule.
Magnetic EEG/EKG Guided-Resonance Therapy (MeRT)
P a g e |
8

FOI 24/25-0151
Reference List
1.
Barahona-Corrêa JB, Velosa A, Chainho A, Lopes R, Oliveira-Maia AJ. Repetitive Transcranial
Magnetic Stimulation for Treatment of Autism Spectrum Disorder: A Systematic Review and Meta-
Analysis. Frontiers in Integrative Neuroscience [Internet]. 2018 2018-July-09; 12(27). Available from:
https://www.frontiersin.org/article/10.3389/fnint.2018.00027.
2.
Finisguerra A, Borgatti R, Urgesi C. Non-invasive Brain Stimulation for the Rehabilitation of
Children and Adolescents With Neurodevelopmental Disorders: A Systematic Review. Frontiers in
Psychology [Internet]. 2019 2019-February-06; 10(135). Available from:
https://www.frontiersin.org/article/10.3389/fpsyg.2019.00135.
3.
Khaleghi A, Zarafshan H, Vand SR, Mohammadi MR. Effects of Non-invasive
Neurostimulation on Autism Spectrum Disorder: A Systematic Review. Clin Psychopharmacol
Neurosci [Internet]. 2020; 18(4):[527-52 pp.]. Available from:
http://www.cpn.or.kr/journal/view.html?doi=10.9758/cpn.2020.18.4.527.
4.
Rajapakse T, Kirton A. Non-invasive brain stimulation in children: Applications and future
directions. Translational Neuroscience [Internet]. 2013 01 Jun. 2013; 4(2):[217 p.]. Available from:
https://www.degruyter.com/view/journals/tnsci/4/2/article-p217.xml.
5.
Janicak PG, Dokucu ME. Transcranial magnetic stimulation for the treatment of major
depression. Neuropsychiatr Dis Treat. 2015;11:1549-60.
6.
Dickinson A, DiStefano C, Senturk D, Jeste SS. Peak alpha frequency is a neural marker of
cognitive function across the autism spectrum. European Journal of Neuroscience. 2018;47(6):643-
51.
7.
Kim A, Taghva A. Improved autism behaviors after noninvasive cerebral transmagnetic
stimulation using customized frequency modulation: fol ow-up mean 24 months. American
Association of Neurological Surgeons; April 5-9, 2014; San Francisco, CA2014.
8.
Taghva A, Silvetz R, Ring A, Kim K-YA, Murphy KT, Liu CY, et al. Magnetic Resonance Therapy
Improves Clinical Phenotype and EEG Alpha Power in Posttraumatic Stress Disorder. Trauma Mon
[Internet]. 2015; 20(4):[e27360-e pp.]. Available from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4727473/.
9.
Taghva A, Jin T, Liu CY, Ring A, Jin Y. Biometrics-Guided Magnetic e-Resonance Therapy
(MeRT) in Post-Traumatic Stress Disorder: A Randomized, Double-Blind, Sham Controlled Trial. No
date.
Magnetic EEG/EKG Guided-Resonance Therapy (MeRT)
P a g e |
9
Document Outline
















