
FOI 24/25-0473
DOCUMENT 2
Research Request – NAPA Therapy
• Is intensive therapy (i.e. NAPA) effective and beneficial/will it lead to
substantial functional improvement/increase independence in task when
compared to other therapeutic approaches?
• For children/participants with a disability
from birth and those that
acquire
injury is there an upper age limit at which further significant
improvement/gain from intensive therapy will taper off/cease?
• What sorts of benefits can be achieved (or are claimed) through NAPA
therapy and as compared to conventional therapy (traditional
weekly/fortnightly programs)?
• How do NAPA conduct therapy: is it collaborative within disciplines or are
participant’s still receiving one on one discipline specific therapy?
• What level of therapy is needed to maintain results/are results maintained
Brief
over the long term?
• Are intensive suitable for adults?
• Is intensive therapy suitable for people with attention/fatigue or cognitive
issues (can they focus for duration of intensive 4-5hours, 5 days x 3 weeks
~60-75hours of therapy)
• Effectiveness in home program uptake from intensives v traditional
therapy?
• What indicators used to determine when person has reached their maximal
level of function and plateau?
• What are the strengths and weaknesses of the NAPA approach to skil s
acquisition, as compared to other forms of therapy?
• What guidelines are available to evaluate or determine when NAPA may be
an appropriate approach?
Date
26/11/2020
Julie s22(1)(a)(ii) (
- irrelev
S enior Technical Advisor – TAB)
Requester Katrin s22(1)(a)(ii) - irre (A ssistant Director – TAB)
Researcher Jane s22(1)(a)(ii) - irreleva(R esearch Team Leader - TAB)
Cleared by Jane s22(1)(a)(ii) - irreleva(R esearch Team Leader - TAB)
N A P A T h e r a p y R e s e a r c h
P a g e |
1
Page 30 of 153

FOI 24/25-0473
Contents
Key points ................................................................................................................................................ 3
What is NAPA? ........................................................................................................................................ 4
Intensive therapy .................................................................................................................................... 4
Difference between intensive therapy as described in the literature and NAPA therapy ................. 5
Suit therapy ............................................................................................................................................. 6
Cuevas Medek Exercise ........................................................................................................................... 8
Home based programs ............................................................................................................................ 9
Neuroplasticity and Gross Motor Function Classification Scores ......................................................... 10
Progressive disorders ............................................................................................................................ 14
Congenital neuromuscular disorders ................................................................................................ 14
Physical therapy interventions...................................................................................................... 15
Neurodegenerative Disorders in Childhood ..................................................................................... 15
Reference List ........................................................................................................................................ 30
Please note:
The research and literature reviews col ated by our TAB Research Team are not to be shared
external to the Branch. These are for internal TAB use only and are intended to assist our advisors
with their reasonable and necessary decision making.
Delegates have access to a wide variety of comprehensive guidance material. If Delegates require
further information on access or planning matters they are to call the TAPS line for advice.
The Research Team are unable to ensure that the information listed below provides an accurate &
up-to-date snapshot of these matters
N A P A T h e r a p y R e s e a r c h
P a g e |
2
Page 31 of 153

FOI 24/25-0473
Key points
The NAPA centre does not provide any guidelines or specifics around how they determine which
interventions are delivered during their “intensive model of therapy,” or how they’re implemented
(multidisciplinary or individual therapists?). The centre promotes that its therapy is “highly effective”
and “cutting edge”, but without any protocols or published evidence to substantiate these claims it
is near impossible to determine whether the program is effective and beneficial. Without any
published evidence we can’t know:
a) Which diagnosis or ages this intervention is suitable for
b) What the long term results are or adverse effects (if any)
c) What the appropriate dosage/intensity is, or
d) When the patient has reached their maximal level of function
Based on the information provided on the NAPA website it is clear that the Therasuit, SpiderCage
and Cuevas Medek Exercises are the key interventions delivered during the intensive program.
Current literature does not support these interventions as best practice for cerebral palsy or ‘other’
neurological conditions.
There are intensive interventions (delivered >3 times a week) for cerebral palsy that are supported
by the literature. These include resistance/strength training and interventions for upper limb
function such as Constraint Induced Movement Therapy and Bimanual Training. However, these can
be delivered in a patient’s home or normal environment which makes them highly feasible (and
likely cost effective) – rather than attending a clinic for 2-6 hours a day or 3 weeks. Furthermore,
systematic reviews comparing conventional therapy (1-2 times a week) to more intensive
intervention have reported no clinically meaningful difference.
It is not clear from the NAPA website how patients are fol owed up after their intensive model of
therapy or whether home programs are developed to consolidate any improvements. This is of
concern given that home based programs have been shown in the literature to be highly beneficial.
The NAPA centre does offer weekly therapy sessions (1 hr) with physiotherapists, occupational
therapists and speech pathologists, however, this would only be appropriate for those who live in
Sydney, and it is unclear whether these weekly sessions consist of conventional/best practice
therapy or those delivered in the intensive model.
Information is provided within the document on neuroplasticity and motor function curves for
children with CP. These enable prognosis of gross motor progress across all 5 levels of Gross Motor
Function Classification System levels for ages 0 to 15.
Research update (05/03/2021)
A single systematic review and meta-analysis on garment/suit therapy has been added to the
literature review. The findings of this study do not change the advice/outcomes of the original
research document. Wells, Marquez [1] concluded
“Whilst there is some evidence for the use of
garment therapy it is not sufficiently robust to recommend the prescription of garment therapy
instead of, or as an adjunct to conventional therapy options”.
N A P A T h e r a p y R e s e a r c h
P a g e |
3
Page 32 of 153

FOI 24/25-0473
What is NAPA?
The Neurological and Physical Abilitation or “NAPA” centre uses what they cal the ‘Intensive Model
of Therapy’ (IMOT) when treating children with cerebral palsy (CP) and ‘other’ neurological
disorders. Programs are customised for each patient and vary in time, duration, intensity and tools
used. The program usually consists of 2-6 hours of treatment a day, 5 days a week over 3 weeks. This
wil depend on diagnosis, age, stamina, strengths/weaknesses, and ‘other’ factors.
The core interventions used are the NeuroSuit and Multifunctional Therapy Unit (SpiderCage) in the
intensive therapy programs on children of all ages starting as young as age three. In addition,
therapists deliver Cuevas Medek Exercise (CME).
It is claimed that their methods are
“highly effective” and
“children often advance to the next
developmental skil or higher during the three-week program. For example, if a child is using a
walker, it is not uncommon for them to gain the strength, balance and ability to walk with crutches.”
The centre also provides
• Weekly therapy - available for physiotherapy, CME/MEDEK, occupational
therapy, NeuroSuit (min. 2 hours), and speech therapy.
Fortnightly appointments are not
available.
• VitalStim swallowing therapy
• Developmental feeding therapy
• Speech therapy
• Telehealth – only available to patients with current therapy authorisation with NAPA are
eligible
Intensive therapy
Intensive interventions for children with CP refers to the frequency and amount of training, the
duration of the training session (minutes or hours), and the duration of the training period (weeks or
months). [2, 3] The typical frequency of physical therapy for children with CP in an outpatient setting
is not well documented, however, physiotherapy sessions are typically offered 1-2 times per week to
young children with CP as reported in Norway, Canada and the US. [2, 4] Various studies
investigating intensive therapy/training have typically considered 3 or more sessions per week to
constitute ‘intensive’ compared to conventional treatment. [2, 5]
Although it has been hypothesized that the effectiveness of conventional therapy in children with CP
may depend on the dosage of treatment (i.e. with intensive regimens being more effective), this
assumption is far from proven. Various systematic reviews and meta-analyses (moderate to high
quality) have been published that investigate dosages required to obtain improvements [6] and have
compared conventional to intensive therapy [2, 5, 7] (see Table 1 for more in-depth data). The main
outcomes from these reviews are as follows:
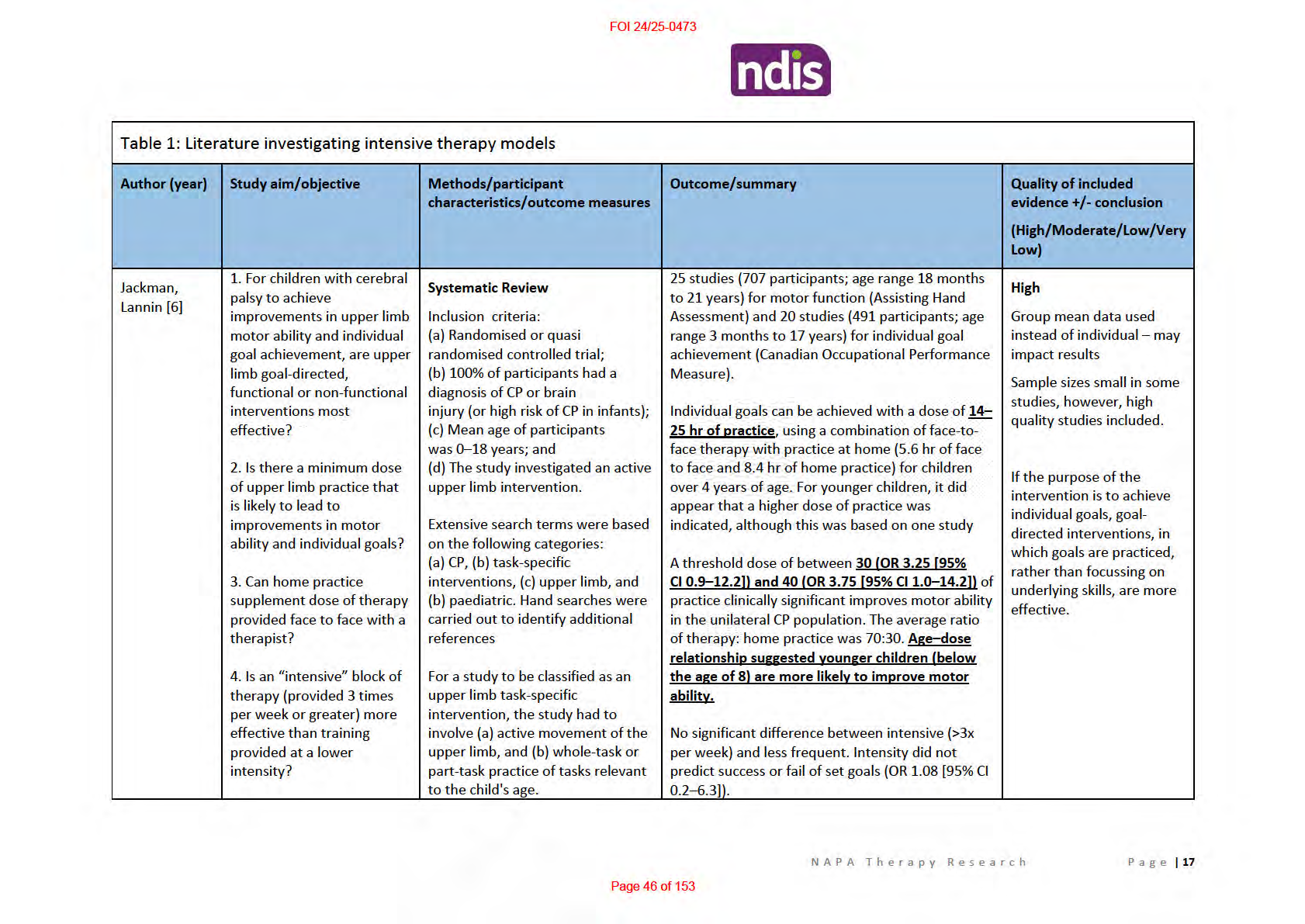
In relation to upper extremity therapy [6]
• Individual goals can be achieved with a dose of
14–25 hr of practice, using a combination of
face-to-face therapy with practice at home (5.6 hr of face to face and 8.4 hr of home
practice) for children over 4 years of age
N A P A T h e r a p y R e s e a r c h
P a g e |
4
Page 33 of 153

FOI 24/25-0473
• A threshold dose of between
30 (OR 3.25 [95% CI 0.9–12.2]) and
40 hrs (OR 3.75 [95% CI
1.0–14.2]) of practice clinically improves motor ability in the unilateral CP population. The
average ratio of therapy: home practice was 70:30.
o Age–dose relationship suggested younger children
(below the age of 8) are more
likely to improve motor ability.
• No significant difference between intensive (>3x per week) and less frequent. Intensity did
not predict success or fail of set goals (OR 1.08 [95% CI 0.2–6.3]).
*It is reasonable to assume that these figures can be transferred to other goal based functional tasks
of the lower extremities.
Comparison of conventional to intensive treatment which target motor and functional skills
(delivered by occupational therapist, physical therapist and/or physiotherapist) showed mixed
results.
• Myrhaug, Østensjø [2] found that across the majority of studies included in their review,
equal improvements were identified between intensive intervention and conventional
therapy or between two different intensive interventions.
• Alternatively, Cope and Mohn-Johnsen [7] and Arpino, Vescio [5] found smal positive
treatment effects in favour of intensive therapy, however, based on the GMFM-88 manual
the level of difference is
not considered clinical y important/noticeable. [8]
Taking a closer look at some of the high quality randomised controlled trials included in these meta-
analyses it is clear that intensive and standard treatment can both lead to improvements in GMFM.
Given that long term follow-up data is sparsely reported, and conventional treatment of 1-2 sessions
per week still leads to significant improvements in motor and functional skills it is difficult to justify
intensive treatment which is more costly, time consuming and tiring/stressful for children. [5]
In addition, there is research of reasonably low to moderate quality which looks at the potential
benefits of intensive strength training. For example, strengthening programs with frequencies of up
to 3 times a week demonstrate improvements in gait and function. [9-13] Protocols have more
commonly been home/community based [9, 11, 12] and have reported changes in gross motor
function [9, 11, 13] cadence, and walking speed. [9, 12, 13] Although these results are positive (and
strength training is wel recognised as a high quality treatment for CP), many studies did not include
a control group to allow for comparison against lower dosages.
Difference between intensive therapy as described in the literature and NAPA therapy
Whilst there are positive findings in the literature (although rarely clinically important or shown to
be sustained over the long term) relating to various types of intensive therapy, we must consider
how this compares to the method proposed by NAPA.
The NAPA program usually consists of 2-6 hours of treatment a day, 5 days a week over 3 weeks. The
vast majority of the literature investigating intensive interventions consists of 3-5 sessions (45-60
minutes in duration) a week over 5-12 weeks. The only other treatment which promotes a dosage as
N A P A T h e r a p y R e s e a r c h
P a g e |
5
Page 34 of 153


FOI 24/25-0473
high as NAPA therapy is Constraint Induced Movement Therapy (CIMT) which has been shown to
range between 1 to 24 hours a day, over a period of two weeks to two months, however, much of
this is parent led/home practice. CIMT is a recommended treatment option as it has an immense
amount of favourable high quality published literature and a good safety profile for those with a
diagnosis of CP.[14, 15] In comparison, NAPA therapy utilises a “core combination” of the Neurosuit,
SpiderCage and Cuevas Medek Exercise (CME). None of which are considered effective (‘do it’ or
‘probably do it’) interventions for CP. [14]
Suit therapy
The original suit (Adeli suit) was developed for the Soviet space program in the late 1960’s and was
referred to as the Penguin suit. It was designed to counteract the adverse effects of zero gravity
including muscle atrophy and osteopenia, and maintain neuromuscular fitness during
weightlessness. [16] In 1991, the Adeli suit incorporated a prototype of a device developed in Russia
for children with CP and popularized by the EuroMed Rehabilitation Center in Mielno, Poland. [17]
Since then, the suit has been popularised in different countries using different names (Therasuit,
Neurosuit, PediaSuit etc.). [16, 18] These different suits are essential y the same thing, however,
they are marketed according to their own ‘protocols’. The differences between these ‘protocols’ are
not clear in the literature, and most interventions use a combination of suits with intensive physical
therapy (i.e. 2-4 hr sessions, 5-6 days a week, over 3 or 4 weeks). [18] Non-peer reviewed literature
from developers of these suits claim that the therapy is appropriate for children from 2 years of age
to adulthood. [18, 19]
In addition to the suit, some protocols use ability exercise units or functional cages. These cages can
be used in two ways: the ‘monkey cage’ uses a system of pul eys and weights to isolate and
strengthen specific muscles; and the ‘spider cage’ (Figure 1) uses a belt and bungee cords to either
assist upright positioning or practice many other activities that normal y would require the support
of more therapists. [18] Claims of “significant improvement” following body weight suspension
training have been made, however, only 3 peer reviewed articles exist. All of which are
N A P A T h e r a p y R e s e a r c h
P a g e |
6
Page 35 of 153

FOI 24/25-0473
methodologically weak and include small samples making it impossible to make conclusions about
its effectiveness. [20-22]
Figure 1. Spider cage and universal therapy unit.
Some of the many reported benefits include improving motor function and posture, [23] improving
vertical stability (e.g. standing posture), [24] increasing range of motion, [25] providing
proprioceptive input and improving the vestibular system improving symmetry, [26] increasing
walking speed and cadence, [27] improving trunk, [28] control motor function (in all dimensions of
Gross Motor Function Measure [GMFM]), [29] and self-care [30] capacity in children with CP.
However, most of these studies are case reports or descriptive studies in which the methodological
quality limits the possibility of supporting or rejecting the use of the suit therapy in clinical settings.
Centres that offer suit therapy indicate that the therapy can help children diagnosed with: [31, 32]
• Cerebral Palsy
• Global Developmental Delays
• Traumatic Brain Injury
• Near Drowning Accidents
• Post stroke (CVA)
• Incomplete Spinal Cord Injury
• Ataxia
• Athetosis
• Spasticity
• Hypotonia
• Parkinson Disease
• Chromosomal Disorders
• Autism Spectrum Disorder
There are no published, peer-reviewed studies on any of the above listed diagnoses, except for CP.
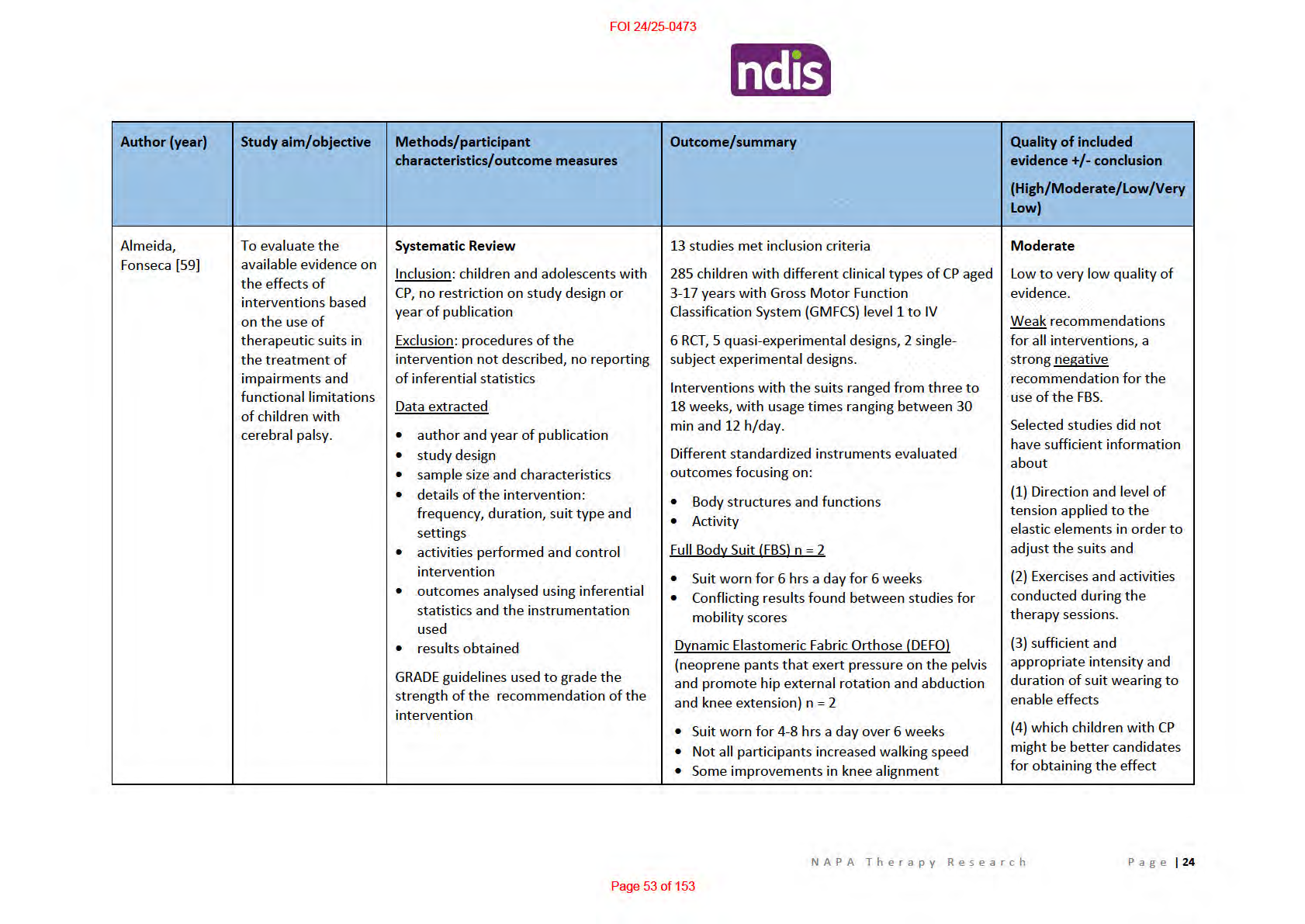
Three moderate to high quality systematic reviews were analysed to obtain evidence on the benefit
of participation in intensive suit therapy for children and adolescents with CP. These reviews are
summarised in Table 2 below.
The main take-home messages from the analysis were:
• Evidence indicating greater functional benefit from participation in intensive suit therapy is
limited.
• No studies investigated the feasibility (e.g. adherence/compliance) or cost-effectiveness of
suit therapy
• It is not possible to draw conclusions regarding which children with CP may benefit more
than others from suit therapies due to the limited evidence and heterogeneity of included
participants (GMFCS level I-IV)
• There is no consensus with regard to frequency, intensity and timing due to the variability in
doses delivered across studies. Often specific protocols (including other physical therapy
interventions concurrently delivered) were not described in studies. This makes it extremely
difficult to evaluate findings.
N A P A T h e r a p y R e s e a r c h
P a g e |
7
Page 36 of 153

FOI 24/25-0473
• Results from a meta-analysis showed a
smal positive effect size for gross motor function at
post treatment (g=0.46, 95% confidence interval [CI] 0.10–0.82) and follow-up (g=0.47, 95%
CI 0.03– 0.90). This small effect
does not support robust conclusions to prescribe or suggest
this new and ‘promising’ approach to therapy.
• Furthermore, adverse effects such as overheating, respiratory compromise, toileting
problems such as constipation and urinary leakage and peripheral cyanosis have been
reported. [16, 33]
Cuevas Medek Exercise
Cuevas Medek Exercise (CME) is a specialised psychomotor therapy designed for infants with
developmental delays, syndromes and conditions affecting the central nervous system. [34] CME
therapy provokes the child's automatic postural responses by exposing the infant to the influence of
gravity through a variety of positions and exercises (approximately 3000 exercises exist). During
CME, the therapist physically manipulates the child to stretch out tight muscles and train the
muscles in groups. These manipulations eventually allow the child to gain control over his or her
trunk, which is necessary to perform basic gross motor activities such as sitting, standing, and
walking. Sessions begin on a table. Then, if the child is able to stand with ankle support, the floor is
used. Floor exercises involve seven pieces of equipment, which can be configured in various ways to
challenge the child’s sense of balance. Exercises are repeated until the reaction of the brain becomes
automatic and the body reacts normal y to situations where required to keep its balance.
It should be noted that CME rejects the use of external supports (splints and walkers) and the
exercises are manually applied by a therapist, rather than the patient having to physical y make the
movements themselves. Below is an excerpt from the thesis titled
The social construction of
disability and the modern-day healer by Vanderminden [35]which describes the process of CME as
described by its creator, Ramon Cuevas.
“CME therapy can be exercised regardless of the emotional status of the child, while in classical
approaches, if the child cries the therapy session is typically terminated. When considering a child's
muscle tone, classic approaches generally wil not place a child with hyper tonicity or severe
spasticity in the standing position. Conversely, CME therapy practices the exact opposite. CME
therapy does not require a physician's diagnosis of a child's condition, but rather seeks to listen to the
parent's interpretation of the limitations of their child's development and movement.” CME is claimed to be suitable for babies from 4 months old, until they are walking and climbing
stairs, however due to the nature of the technique, therapists are only able to work with children of
a certain weight (up to approximately 22.5kg/50 pounds). [34] The therapy is suggested to occur
three times a week, twice a day, for 45 minutes per session. [36]
Studies focused on CME are scarce. Apart from reports published by the creator of the technique,
only two case reports published in very low ranked (Impact Factor <1.5) peer reviewed journals
could be located. [36, 37] These studies report that technique leads to positive results, however,
several factors need to be considered.
1) Treatment protocols were poorly reported
N A P A T h e r a p y R e s e a r c h
P a g e |
8
Page 37 of 153

FOI 24/25-0473
2) Unclear what outcome measures were used to determine “positive” results
3) No statistical analysis
4) Small samples/case reports
a. Unclear how participants were selected and allocated to groups in the report with
multiple participants [37]
Given the lack of scientific evidence or identification of possible adverse effects of this treatment it
cannot be considered an evidence based practice. It should also be noted that CME is not listed as an
intervention for CP in the high quality systematic review by Novak, Morgan [14] or the American OT
association review into interventions to improve motor performance [38], furthermore, other
authors have cal ed for the treatment to be “discontinued based on current evidence.” [39]
Home based programs
Home programs have been used for years by families and therapists to increase the intensity of
therapy, either between treatment sessions or during a break from therapy. Recent research into
therapy intensity has concluded that home programs provide a pragmatic solution to achieving high
dose therapy, thus overcoming existing systemic implementation barriers.[2, 40]
In relation to upper limb mobility, there is little evidence to support block therapy alone as the dose
of intervention is unlikely to be sufficient to lead to sustained changes in outcomes. [40] There is
strong evidence that goal-directed OT home programs are effective and could supplement hands-on
direct therapy to achieve increased dose of intervention. [41] Embedding intervention in natural
environments (e.g., home, preschool/school) has been suggested to lead to meaningful and
generalizable improvements in function. [42]
Clinically proven high dose interventions such as bimanual training and constraint-induced
movement therapy (CIMT) have been shown to be effective when delivered at home. [43-45] Home
based interventions are beneficial, especially for interventions with dosages that are not feasible for
most families.
Novak, Cusick [42] have developed five steps for delivering successful home based programs. This
includes:
1) Establishing collaborative partnerships between therapist and caregivers
2) Having the child and family (not the therapist) set goals about what they would like to work
on in the home environment
3) Establishing the home program by choosing evidence based interventions that match the
child and family goals and empowering the parents to devise or exchange the activities to
match the child’s preferences and the unique family routine
4) Providing regular support and coaching to the family to identify the child’s improvements
and adjust the complexity of the program as needed; and
5) Evaluating the outcomes together
Based on the steps, therapy provided by NAPA would not be successful in a home based
environment because:
N A P A T h e r a p y R e s e a r c h
P a g e |
9
Page 38 of 153

FOI 24/25-0473
a) The core interventions (suit therapy, spider cage and CME) are not evidence based
b) They cannot be performed in the home without the equipment utilised in the clinic
c) The NAPA centre is not local for many patients so provision of support and development of a
collaborative partnership will be near impossible without regular interaction between
therapists and patients/families
d) Outcomes won’t be able to be evaluated unless further blocks of NAPA therapy are provided
Neuroplasticity and Gross Motor Function Classification Scores
Neuroplasticity is the brain’s adaptive capacity to encode experiences as well as learn new
behaviours and skills. In children with CP, intervention before the age of seven is recommended for
optimizing motor function and learning functional skills, because from a maturational and
neuroplasticity perspective the greatest gains wil be made during this window. [46-48]
A younger child with a GMFCS level I or II usually has a better developmental prognosis than an older
child with a GMFCS level IV or V. [49]
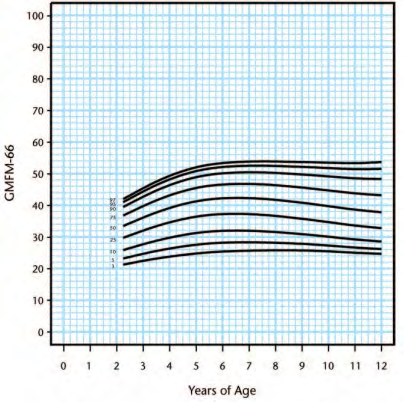
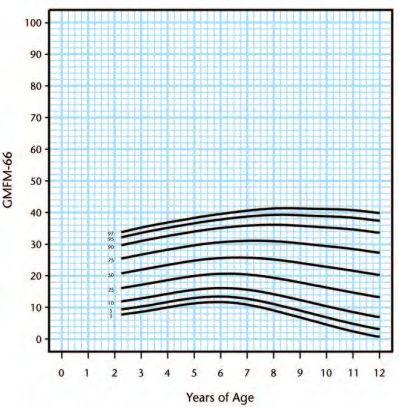
Gross motor development curves based on age and GMFCS level have been created by Rosenbaum,
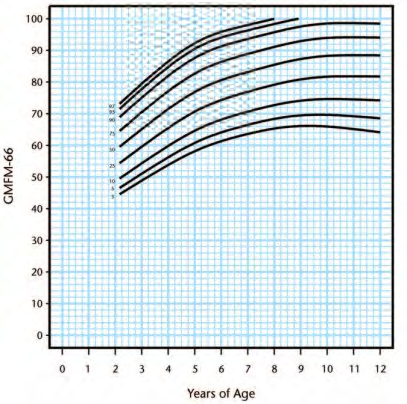
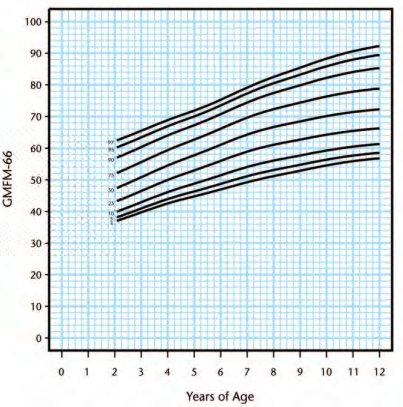
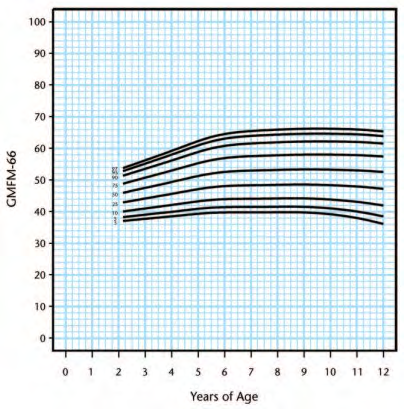
Walter [46] to enable prognosis of gross motor progress (Figure 2). Following this, Hanna, Bartlett
[50] created reference curves which plotted percentiles at the 3rd, 5th, 10th, 25th, 50th, 75th, 90th,
95th, and 97th percentiles within each GMFCS level (Figure 3-7). This can be used to determine
percentage potential based using GMFCS scores.
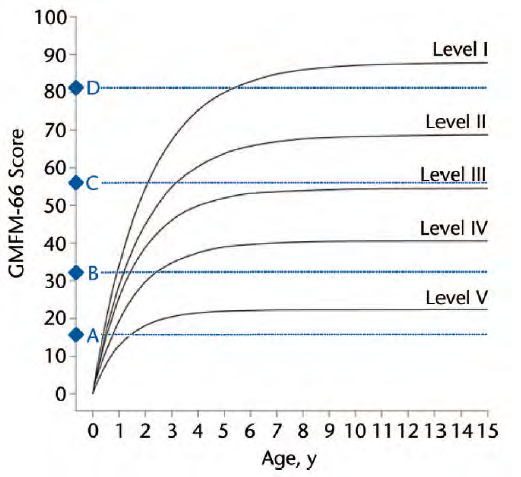
Figure 2. Gross motor development curves representing average development predicted by the Gross Motor
Classification System. The diamonds on the vertical axis identify 4 items of the 66-item Gross Motor Function
N A P A T h e r a p y R e s e a r c h
P a g e |
10
Page 39 of 153

FOI 24/25-0473
Measure (GMFM-66) that predict when children are expected to have a 50% chance of completing that item
successful y. The GMFM-66 item 21 (diamond A) assesses whether a child can lift and maintain his or her head
in a vertical position with trunk support by a therapist while sitting, item 24 (diamond B) assesses whether a
child can maintain a sitting position on a mat without support from his or her arms for 3 seconds, item 69
(diamond C) measures a child’s ability to walk forward 10 steps without support, and item 87 (diamond D)
assesses the task of walking down 4 steps by alternating feet with arms free.
N A P A T h e r a p y R e s e a r c h
P a g e |
11
Page 40 of 153

FOI 24/25-0473
Figure 3. Gross Motor Function Classification System level I percentiles.
Figure 4. Gross Motor Function Classification System level II percentiles
N A P A T h e r a p y R e s e a r c h
P a g e |
12
Page 41 of 153

FOI 24/25-0473
Figure 5. Gross Motor Function Classification System level III percentiles
Figure 6. Gross Motor Function Classification System level IV percentiles
N A P A T h e r a p y R e s e a r c h
P a g e |
13
Page 42 of 153

FOI 24/25-0473
Figure 7. Gross Motor Function Classification System level V percentiles
Progressive disorders
Childhood neurodegenerative and neuromuscular disorders are rare, and usual y have no cure. The
natural history is often unknown and progression varies across patients.
Congenital neuromuscular disorders
Congenital neuromuscular disorders include:
• Muscular dystrophy
• Myotonic dystrophy
• Spinal muscular atrophy
• Peripheral neuropathies
• Generalised muscle and nerve issues (such as mitochondrial disorders)
The management of paediatric neuromuscular disorders is complex and challenging. Developing an
effective management plan requires an understanding of the underlying pathophysiology, genetics,
and natural history, as well as the interactions of normal maturation, treatment modalities, and the
N A P A T h e r a p y R e s e a r c h
P a g e |
14
Page 43 of 153


FOI 24/25-0473
environment. [51, 52] Optimum management requires a multidisciplinary approach that focuses on
preventive measures as well as active interventions to address the primary and secondary aspects of
the disorder. [51]
Physical therapy interventions
Active, active-assisted, and/or passive stretching to prevent or minimise contractures should be
done a minimum of 4–6 days per week for any specific joint or muscle group. Stretching should be
done at home and/ or school, as well as in the clinic. [51]
Nowhere in the literature is there mention
of providing short-term intensive therapy (physio, OT or speech) blocks as part of the
management plan for neuromuscular disorders. Figure X below provides a comprehensive overview of neuromuscular and skeletal management
strategies for Duchenne muscular dystrophy. [51]
Figure X. neuromuscular and skeletal management strategies for Duchenne muscular dystrophy
Neurodegenerative Disorders in Childhood
Normal neural development and behaviour is relatively wel understood, much less is known about
the behavioural neurology of neurodegenerative deterioration in children. [53] It is unknown how
the developing brain is impacted by progressive diseases at both a global and selective level. [53, 54]
Frequently, the assessment of the severity of symptoms in children with neurodegenerative
N A P A T h e r a p y R e s e a r c h
P a g e |
15
Page 44 of 153

FOI 24/25-0473
disorders (NDD) is difficult. Age and understanding of the child are always a factor and, in addition,
many children suffer from brain damage or intellectual disability as a result of their disease. [53]
Treatment of children with NDD is directed towards the underlying disorder, other associated
features, and complications. [55] The treatable complications include; epilepsy, sleep disorder,
behavioural symptoms, feeding difficulties, gastroesophageal reflux, spasticity, drooling, skeletal
deformities, and recurrent chest infections. [55] These children require a multidisciplinary team
approach with the involvement of several specialties including paediatrics, neurology, genetics,
orthopaedics, physiotherapy, and occupational therapy. [55] Many newer antiepileptic drugs are
now available to treat intractable epilepsy. [54]
owhere in the literature is there mention of
providing short-term intensive therapy (physio, OT or speech) blocks as part of the management
plan for NDD. An investigation by Olney, Doernberg [56] identifed 104 progressive brain disorders of childhood
which may be mistaken for CP. The natural history of many of these conditions is unknown as
insuffient numbers of cases are reported in the literature. [56]
N A P A T h e r a p y R e s e a r c h
P a g e |
16
Page 45 of 153

FOI 24/25-0473
5. Is there an age–dose
relationship?
Outcome measures
Practice at home appears to be an effective
AHA, Quality of Upper Extremity
enhancement to face-to-face therapy. It is likely
Skil s Test (QUEST), Melbourne
that if families are educated and supported to
Assessment 2, The Box and Blocks
carry out practice at home, that this practice can
Test, Abilhand-Kids, COPM, The
be an effective and cost-effective enhancement in
Goal Attainment Scale (GAS), the
achieving goals. Practice within everyday
Pediatric Evaluation of Disability
environments may also facilitate transfer of skil s
Inventory (PEDI), and the
beyond the clinic to the child's real life.
Functional Independence Measure
for children (WeeFIM). The AHA
and COPM were the most
commonly utilised reliable
outcome measures within eligible
studies
Cope and
(1) In children with cerebral
Systematic Review & Meta-
9 RCTs and 1 retrospective non-randomized
Moderate
Mohn-
palsy, is therapy provided
Analysis
control ed trial (388 participants, age 4 months to
Johnsen [7]
for a greater total number
16 years)
Methods of review were
of minutes more effective
inclusion criteria: Study design
robust. Included studies
than the same intervention
must include the same treatment
The functional level of the participants ranged
highly variable.
provided at fewer total
across at least one of three
from I to V on the GMFCS. The majority of
minutes for improving
dosage variables, specifical y: (a)
participants throughout the studies included
motor function? (Time)
compare treatment time, defined
children with spastic cerebral palsy.
Not enough evidence exists
for this review as any contrast
to determine if higher
(2) In children with cerebral in the total number of minutes; (b) The majority (8 of 10) of studies utilized either an
frequency therapy is more
palsy, is therapy provided at compare treatment frequency,
eclectic (treatment not limited to one specific
effective than lower
higher frequency
defined for this study as any
intervention) or neurodevelopmental treatment
frequency.
(intermittent) more
contrast in scheduling of frequency (NDT) approach
effective than the same
(intermittent versus continuous)
intervention provided at a
where total minutes of therapy
The high-dosage therapy conditions ranged in
The findings from this
lower frequency
remain constant; and (c) compare
frequency from one to seven times per week, with review are limited to short-
(continuous) for improving
intensity in which the amount of
total therapy hours over the treatment duration
term effects only; fol ow-
motor function?
effort by the study participant is
ranging from 9 to 126 hours. Low-dosage therapy
up data were sparsely
(Frequency)
varied by group;
conditions ranged in frequency from one time per
reported.
month to seven times per week, with total therapy
N A P A T h e r a p y R e s e a r c h
P a g e |
18
Page 47 of 153

FOI 24/25-0473
(3) In children with cerebral intervention must be provided by a hours over the treatment duration ranging from 6
palsy, is intervention
PT or OT intervention may focus on to 78 hours.
performed at a higher
upper and/or lower limb; outcomes
intensity more effective
measures include impairments of
Results showed a
smal treatment effect favouring
than the same intervention
body structure/ function, activity
the higher dosage time (pooled g = 0.277, 95% CI
performed at a lower
limitations, and/or participation
0.02, 0.534; I2 = 0%), however, this benefit is
not
intensity for improving
restrictions; participants must be
clinically important.
motor function? (Intensity)
children, birth to 18 years with a
diagnosis of cerebral palsy;
Al individual between group differences showed
publication in peer-reviewed
wide confidence intervals that crossed zero,
journals in any language with
suggesting both lack of precision in the computed
English version available;
effect sizes and the possibility that there was no
control ed trials with two or more
difference between the groups.
groups.
Data extracted
study design, sample size, subject
demographics, intervention
parameters, outcome measures,
fol ow up procedures, baseline and
post treatment group means and
measures of variability, within-
group change scores and measures
of variability, and statistical
significance for within group and
between-group comparisons
Myrhaug,
To describe and categorise
Systematic review & Meta-
38 studies included 1407 children with al levels of
Moderate
Østensjø [2]
intensive motor function
gross and fine motor function
and functional skil s training
Analysis
Smal studies, often
among young children with
Inclusion criteria: (a) a study
Only 6/38 studies performed intervention more
without power
CP, and to summarise the
population of CP with a mean age
than 1 hr a day. More common for 2-7 sessions a
calculations, were also
effects of these
<7 years; (b) evaluated the effects
week + home training (19/38) and these were
included. A variety of
interventions.
of motor function (e.g., mobility
mainly hand function interventions.
interventions were used to
and grasping) and functional skil s
improve gross motor
training (e.g., eating and playing)
In a majority of the studies, equal improvements in function and functional
performed three times or more per motor function and functional skil s were identified skil s, which prevented the
N A P A T h e r a p y R e s e a r c h
P a g e |
19
Page 48 of 153

FOI 24/25-0473
week at the clinic, in the
for intensive interventions and conventional
pooling of results in the
kindergarten, or at home; (c) was
therapy or between two different intensive
meta-analyses.
compared to another intervention
interventions
19/38 studies had high risk
(e.g., conventional therapy), the
of bias. Therefore, results
same type of intervention provided
Hand function (fine motor skills)
remain uncertain.
less frequently, or another
When compared with conventional therapy, CIMT
intensive intervention; and (d) with performed for more than one hour per day showed The identification of the
outcomes in the activity and
significant effects on unilateral hand function in
optimal intensity of
participation components of the
one meta-analysis (N = 2, [33,60] SMD 0.79 (95% CI interventions that target
ICF [3], measured as hand function, 0.03, 1.55), p = 0.04). The CIMT groups performed
motor function and
gross motor function, and/or
15–28 hours more training per week, which
functional skil s, as wel as
functional skil s.
resulted in a difference of 29–84 training hours
the possible harmful
over two to three weeks compared with the
effects of intensive
In addition, the included studies
conventional therapy groups.
training, requires further
were required to be control ed
investigation.
trials, published in peer review
Gross motor function
journals
Too heterogeneous to be pooled in meta-analyses.
All studies with significant results in favour of
Data extracted
intensive training that targeted gross motor
study population, design,
function had a high risk of bias.
interventions, comparison,
outcome measures, and results
Functional skills
CIMT performed at least 2–7 sessions per week
The intensity of training was
with additional home training achieved more
described as the amount of training improvements in functional skil s compared with
and duration of the training
conventional therapy (N = 3, [36,38,60] SMD 0.82
periods. The amount was
(95% CI 0.26, 1.38), p = 0.004) and (2) CIMT
categorised into four groups
performed 2–7 sessions per week with additional
according to frequency of sessions
home training achieved more improvements
and use of home training: (1) 2–7
in functional skil s compared with intensive
training sessions per week with
bimanual home training (N = 4, [21,30,32,34] SMD
additional home training, (2) 3–7
0.50 (95% CI 0.16, 0.83), p = 0.004)
training sessions per week, (3)
training more than one hour per
day, and (4) training more than one
N A P A T h e r a p y R e s e a r c h
P a g e |
20
Page 49 of 153

FOI 24/25-0473
hour per day with additional home
training.
The duration was categorised as ≤
four weeks, 5–12 weeks, or >12
weeks.
Arpino, Vescio To assess whether intensive
Systematic review & Meta-
Meta-analysis showed that the GMFM change
High
[5]
‘conventional therapy’ is
score was higher for the intensive treatment
more effective than non-
analysis
group, compared with the non-intensive treatment
According to the
intensive ‘conventional
Type of study: RCT
group
[difference of 1.32; 95% confidence interval GMFM-88 manual an
therapy’ in children with CP
(CI): 0.55–2.10].
increase of 1.82% points is
whose clinical outcome was Type of participants:
the smal est change of
assessed with the GMFM.
infant/children/adolescents (1–18
Effect of intensive treatment tended to be stronger
clinical importance
years old) affected by any type of
for children who were 2 years of age or younger
according to parents’
CP.
(difference of 5; 95% CI: – 0.45–10.45).
perception
Outcome measure: GMFM.
In the RCTs in which treatment lasted for at least
Limited evidence to
‘intensive’ treatment was defined
60 days, it was higher in the intensive treatment
support
as any treatment provided more
group than in the non-intensive treatment group
intensive/additional
than 3 times per week; in a single
(difference of
1.42; 95% CI: 0.55–2.30).
physiotherapy
study, additional sessions provided
by an assistant defined the
‘intensity’ of the treatment.
‘Conventional therapy’ that which
included physiotherapy or a
neurodevelopmental approach.
Elgawish and
To assess gross motor
Randomised control ed trial
After 8 weeks, there were significant differences
Moderate
Zakaria [41]
progress in children with
between the two groups as regards the total
spastic (quadriplegic and
Patients were randomly assigned
scores of GMFM-88 and GMPM (
P < 0.05).
Convenience sample.
diplegic) CP treated with
to two treatment groups: group A
Randomisation not
intensive physical therapy
and group B
However, highly significant differences for
specified, no power
(PT) as compared with a
GMFM-88 (
P < 0.001) and only significant
calculation.
matched group treated with Convenience sample.
differences (
P < 0.05) for GMPM were observed
a standard PT regimen.
after 16 weeks.
Intensive PT = 5 sessions (1hr each)
Intensive PT led to greater
a week, over 16 weeks
motor function
N A P A T h e r a p y R e s e a r c h
P a g e |
21
Page 50 of 153

FOI 24/25-0473
Standard PT =2 sessions (1hr each), No statistical y significant differences were found
improvements. However,
over 16 weeks
between the two groups as regards GMFM-66
even 1hr, twice a week
scores after 8 weeks, and significant differences
leads to significant
25 girls and 20 boys, aged between were found only after 16 weeks (
P < 0.05).
improvements.
2 and 6
Years
After 16 weeks, al dimensions of GMFM-88 were
significantly increased in both groups (P < 0.001).
GMFCS level I - V
Christiansen
to compare the effect of the
Randomised control ed trial
Both groups increased their GMFM scores
Moderate
and Lange
delivery of the same
significantly over the study period (I group
[57]
amount of intermittent
25 children up to 10 years of age
p=0.026; C group p=0.038).
Convenience sample.
versus continuous
(16 males, nine females; median
Randomisation not
physiotherapy given to
age 3y 2mo, range 1y 2mo–8y
Result does not confirm the hypothesis that
specified. More studies
children with cerebral palsy 9mo)
intermittent physiotherapy increases the GMFM-
required.
(
Convenience sample.
66 score more than continuous physiotherapy
GMFCS level I – V
Intermittent = physiotherapy 4x a
week, 45 minutes per session for 4
weeks (period A) fol owed by 6
weeks without physiotherapy
(period B). Periods A and B were
repeated three times over 30
weeks with a maximum of 48
sessions
Continuous = physiotherapy once
or twice a week for 30 weeks, also
for 45 minutes per session and with
a maximum of 48 sessions
Children were treated by ‘their
own’ physiotherapist during the
intervention
Outcome measure
N A P A T h e r a p y R e s e a r c h
P a g e |
22
Page 51 of 153

FOI 24/25-0473
GMFM-66
Bower,
to determine whether
Randomised control ed trial
There was no statistical y significant difference in
Moderate
Michel [58]
motor function and
the scores achieved between intensive and routine
performance is better
A convenience sample of 56
amounts of therapy or between aim-directed and
Randomised, power
enhanced by intensive
children with bilateral CP classified goal-directed therapy in either function or
calculation and good CI
physiotherapy or
at level II or below on the Gross
performance.
estimates.
collaborative goal-setting in Motor Function Classification
children with cerebral palsy System (GMFCS), aged between 3
Intensive physiotherapy, in contrast to
The results of this trial
and 12 years.
collaborative goal-setting, produced a trend
suggest that for children
aged 3 to 12 years with
4 treatment regimens provided by
towards improvement in the GMFM scores which
bilateral CP at levels II or
their own physiotherapist during
was not statistical y significant. This trend declined below on the GMFCS,
the treatment period: (1) current
in the fol ow-up observation period.
altering their routine
pattern of physiotherapy to
physiotherapy by
continue for each child; (2) current
increasing its intensity for a
pattern of physiotherapy to be
period of six months has
provided more intensively, one
very little effect upon the
hour per day Monday to Friday; (3)
outcome of gross motor
therapy to be guided by
function or performance at
col aborative setting of specific,
the end of this time.
individual, and measurable goals at
the current intensity, i.e. amount
as in Group 1; (4) therapy to be
guided by col aborative setting of
specific, individual, and measurable
goals and provided more
intensively, one hour per day
Monday to Friday
Outcome measure
GMFM-88
Table 2. Summary of systematic reviews investigating intensive suit therapy as a treatment for CP
N A P A T h e r a p y R e s e a r c h
P a g e |
23
Page 52 of 153

FOI 24/25-0473
• Wearing this suit did not lead to significant
difference in postural control
TheraTogs n = 3
• Suit worn 12 hrs a day for 12 weeks
• Significant improvement seen in gait kinematics
across all studies compared to control groups
TheraSuit Method (TSM) or AdeliSuit Therapy (AST)
n = 6
• Both Therasuit studies found minimal gain
(smal positive effect sizes).
• No statistical y significant differences between
groups [23, 27]
• Some areas there was a decline in gross motor
function [27]
• Those with higher level motor function at
baseline performed better [23]
• Only care giver perception regarding the
performance of tasks obtained a large effect
size
• Same findings relating to Adeli suit therapy
Karadağ-Saygı
To evaluate the
Systematic Review
29 studies were included of which
Moderate
and Giray [33]
clinical aspects and
10 (34.5%) were Class I, eight were
effectiveness of suit
Inclusion
(27.6%) Class II-I I, and 11 (37.9%) were Class IV
Heterogeneity of studies
therapy for patients
makes it difficult to provide
with cerebral palsy
• Patients: Children (<18 years) with a
diagnosis of CP
Types of participants
any guidance for clinical
•
practice.
• Intervention: Suit therapies
Age ranged between 3 and 14 years.
•
• Comparison: Conventional therapy,
Sample size ranged from 16 to 51.
Smal sample sizes of
neurodevelopmental therapy, or
• Fourteen (48.28%) of the studies did not report included studies and
another therapeutic approach
the GMFCS level of the participants.
varying protocols
Intervention protocols
N A P A T h e r a p y R e s e a r c h
P a g e |
25
Page 54 of 153

FOI 24/25-0473
• Outcome: The clinical aspects of
• Intervention protocols varied within and
Included low quality
studies (number of participants, age,
between studies
studies (case studies etc)
CP type, Gross Motor Function
• Suit designs also differed among studies and
Classification System (GMFCS) level,
varied among study participants in some of the
suit type, intervention including dose
studies
Further studies including
of suit therapy, outcome
• Nine (31.03%) of the studies investigated the
large numbers of
measurements, outcomes, adverse
effect of suit on upper limb function, while 10
children with CP at
effects, and funding)
of them investigated effects on lower limb
different functional levels
• Study: All types of trials published in
function (e.g. gait analysis parameters, balance and ages are required in
peer reviewed journals including
or walking performance tests)
order to establish impact
RCTs and non-RCTs and other studies
in children with CP at
(single case studies or case series)
Types of outcome measures
different functional levels
and ages via subgroup
Data extracted
• The Gross Motor Function Measure was the
most reported outcome
analysis
• Number of participants
• Participation evaluated using the International
• Age
Classification of Functioning, Disability, and
• CP type,
Health were limited
• GMFCS level
• Seventeen (58.62%) of the studies did not
• Suit type
report parental satisfaction or adverse effects.
• Intervention including dose of suit
therapy
Results synthesis
• Outcome measurements
• A single RCT of high quality showed that ful
• Adverse effects
body suit therapy in additional to conventional
therapy is beneficial in improving gross motor
function in diplegic CP
• Moderate quality evidence from 4 RCTs
showed that suit therapy in addition to
conventional therapy yields no significant
change in GMFM compared to conventional
therapy in children with diplegic and tetraplegic
CP.
• None of the studies investigated the feasibility
(e.g adherence/compliance), and cost-
effectiveness.
N A P A T h e r a p y R e s e a r c h
P a g e |
26
Page 55 of 153

FOI 24/25-0473
• Adverse effects were reported in 11 of the
included studies. The reported undesirable
effects were difficulty in donning/doffing,
toileting problems such as constipation and
urinary leakage, decrease in respiratory
function, heat and skin discomfort (e.g.
hyperthermia in summer, cyanosis)
Martins,
An overview of the
Systematic Review & Meta-Analysis
Four studies were eligible and included in the
High
Cordovil [16]
efficacy of suit
review
therapy on
Inclusion criteria
Overal , studies were rated
functioning in
110 participants included
as ‘fair’ to ‘good’ quality
children and
• RCTs reported in peer-review
using the PEDRO scale.
adolescents with
journals
Mean number of participants in each trial was 12.3
cerebral palsy.
• Languages: English, Portuguese,
(SD 2.52) with a mean age of 6 years 11 months
The results of the study
Spanish and French
(SD 1y 10mo).
point to limited effects of
• Studies investigating the effect of
suit therapy in gross motor
suit therapy regardless of the type of
function of children and
• Two RCTs compared Adeli suit treatment with
protocol used (Pedia- Suit, TheraSuit,
adolescents with CP, and
neurodevelopmental treatment (NDT)
NeuroSuit, Adeli suit, Penguin suit, or
considerable levels of
•
Bungy suit);
one study compared modified suit therapy with heterogeneity between
conventional therapy
• Studies conducted with samples that
trials.
comprised children and adolescents
• One compared TheraSuit with a treatment
(from 0–18y) with a clinical diagnosis
categorized as ‘other’
The presence of potential
of CP regardless of the type and level
co-interventions (such as
of severity
Sample
additional interventions
•
• Studies reporting functioning as the
CP severity ranged from I to IV
and home training of
primary outcome, assessed by means • Subtypes included spastic, ataxic and dyskinetic parents with their children)
of standardized and international y
• Topographic distribution of motor signs –
remained unclear in most
accepted instruments (e.g. GMFM –
hemiplegia, diplegia and quadriplegia
studies and might have
66 or 88 items and Paediatric
• Total hours of treatment ranged from 30-60
influenced outcomes.
Evaluation of Disability Inventory
[PEDI]).
There is no consensus
Data extracted
Adeli suit showed significant improvements in
about the adequate
• Type of study design
gross motor function after 1 month of treatment
duration of suit therapy
• Sample size
(p=0.037). However, there was a decrease in gross programs.
motor function at fol ow up (9 months) and not
N A P A T h e r a p y R e s e a r c h
P a g e |
27
Page 56 of 153

FOI 24/25-0473
• Instruments
difference between Adeli suit and NDT when the
• Intervention protocol
retention of motor skil s was tested. This suggests
Health professionals should
• Outcomes
that AST could result in short-term gains quickly,
take into consideration the
although long-term improvements in gross motor
lack of scientific evidence
Methodological quality
function may occur best with traditional NDT
regarding the effectiveness
PEDro scale
methods.
of suit therapy when
advising parents who are
Remaining RCT’s showed variable results:
enquiring about this costly
• 2 showed significant differences between suit
and time-consuming
therapy and conventional/control groups
treatment option.
• 1 showed no difference between TheraSuit and
control suit when delivered as part of an
In summary, the results of
intensive therapy program
this systematic review and
meta-analysis do not
No studies fully specify the type of activities and
support robust conclusions
exercises performed by participants in the
to prescribe or suggest this
experimental conditions who enrol ed in different
new and ‘promising’
protocols of suit therapy, and those in the control
approach to therapy.
conditions.
Meta-Analysis
Small, pooled effect sizes were found for gross
motor function at post treatment (g=0.46, 95%
confidence interval [CI] 0.10–0.82) and fol ow-up
(
g=0.47, 95% CI 0.03– 0.90).
Wells, Marquez To conduct a
Systematic Review & Meta-Analysis
14 studies included in the review (n = 234)
Moderate
[1]
systematic review
asking, does garment Electronic searches of EMBASE,
Age 15 months to 17 years (mean = 8.1 years).
Limited number and
therapy improve
MEDLINE, Cochrane Library, PubMed,
Primary reported impairment was spasticity
varying quality of studies
motor function in
CINAHL and Proquest
(74.76%).
children with
Whilst there is some
cerebral palsy?
Inclusion criteria: Children <18 years,
evidence for the use of
any sub classification of CP, intervention 5 RCT, 9 were case studies (single case study,
garment therapy it is not
involved suit/garment therapy and
repeated measures or case report).
sufficiently robust to
included a measure of neuromuscular
recommend the
function
4 studies full body suits, 6 studies full body suits in
conjunction with a strapping system, 2 upper limb
prescription of garment
N A P A T h e r a p y R e s e a r c h
P a g e |
28
Page 57 of 153

FOI 24/25-0473
garment, 1 study lower limb garment, 1 full body
therapy instead of, or as an
suit including gloves. Garment brands were Second adjunct to conventional
Skin, the Adeli Suit, TheraTogs, TheraSuit, UpSuit,
therapy options.
and Camp Lycra
9 described adverse events that may have been a
consequence of the intervention
Intervention duration = 3-12 weeks
Garment wear time = 2-12 hours per day
Meta-Analysis
Non-significant effect on post-intervention
function as measured by the Gross Motor Function
Measure when compared to controls (MD = −1.9;
95% CI = −6.84, 3.05).
Non-significant improvements in function were
seen long-term (MD = −3.13; 95% CI = −7.57, 1.31).
Garment therapy showed a significant
improvement in proximal kinematics (MD = −5.02;
95% CI = −7.28, −2.76), however significant
improvements were not demonstrated in distal
kinematics (MD = −0.79; 95% CI = −3.08, 1.49).
N A P A T h e r a p y R e s e a r c h
P a g e |
29
Page 58 of 153

FOI 24/25-0473
8.
Russel DJ, Rosenbaum P, Wright M, Avery LM. Gross Motor Function Measure (GMFM-66 &
GMFM-88) users manual. United Kingdom: Mac Keith Press; 2002.
9.
Damiano DL, Abel MF. Functional outcomes of strength training in spastic cerebral palsy.
Archives of Physical Medicine and Rehabilitation [Internet]. 1998 1998/02/01/; 79(2):[119-25 pp.].
Available from: http://www.sciencedirect.com/science/article/pii/S0003999398902878.
10.
Damiano DL, Vaughan CL, Abel ME. Muscle Response to Heavy Resistance Exercise in
Children with Spastic Cerebral Palsy. Developmental Medicine & Child Neurology [Internet]. 1995;
37(8):[731-9 pp.]. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1469-
8749.1995.tb15019.x.
11.
Dodd KJ, Taylor NF, Graham HK. A randomized clinical trial of strength training in young
people with cerebral palsy. Developmental Medicine & Child Neurology [Internet]. 2003;
45(10):[652-7 pp.]. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1469-
8749.2003.tb00866.x.
12.
Eagleton M, Iams A, McDowell J, Morrison R, Evans CL. The Effects of Strength Training on
Gait in Adolescents with Cerebral Palsy. Pediatric Physical Therapy [Internet]. 2004; 16(1). Available
from:
https://journals.lww.com/pedpt/Ful text/2004/01610/The Effects of Strength Training on Gait i
n.5.aspx.
13.
Engsberg JR, Ross SA, Col ins DR. Increasing Ankle Strength to Improve Gait and Function in
Children with Cerebral Palsy: A Pilot Study. Pediatric Physical Therapy [Internet]. 2006; 18(4):[266-75
pp.]. Available from:
https://journals.lww.com/pedpt/Ful text/2006/01840/Increasing Ankle Strength to Improve Gait
and.6.aspx.
14.
Novak I, Morgan C, Fahey M, Finch-Edmondson M, Galea C, Hines A, et al. State of the
Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating
Children with Cerebral Palsy. Current Neurology and Neuroscience Reports [Internet]. 2020
2020/02/21; 20(2):[3 p.]. Available from: https://doi.org/10.1007/s11910-020-1022-z.
15.
Hoare BJ, Wallen MA, Thorley MN, Jackman ML, Carey LM, Imms C. Constraint-induced
movement therapy in children with unilateral cerebral palsy. Cochrane Database of Systematic
Reviews [Internet]. 2019; (4). Available from: https://doi.org//10.1002/14651858.CD004149.pub3.
16.
Martins E, Cordovil R, Oliveira R, Letras S, Lourenço S, Pereira I, et al. Efficacy of suit therapy
on functioning in children and adolescents with cerebral palsy: a systematic review and meta-
analysis. Developmental Medicine & Child Neurology [Internet]. 2016; 58(4):[348-60 pp.]. Available
from: https://onlinelibrary.wiley.com/doi/abs/10.1111/dmcn.12988.
17.
Turner AE. The efficacy of Adeli suit treatment in children with cerebral palsy.
Developmental Medicine & Child Neurology [Internet]. 2006; 48(5):[324- pp.]. Available from:
https://www.cambridge.org/core/article/efficacy-of-adeli-suit-treatment-in-children-with-cerebral-
palsy/75AFA67F81E658CD64D18773CC5E438E.
18.
Scheeren EM, Mascarenhas LPG, Chiarello CR, Costin ACMS, Oliveira L, Neves EB. Description
of the Pediasuit ProtocolTM. Fisioterapia em Movimento [Internet]. 2012; 25:[473-80 pp.]. Available
from: http://www.scielo.br/scielo.php?script=sci arttext&pid=S0103-
51502012000300002&nrm=iso.
19.
Koscielny R. Strength training and CP. Available from:
http://www.suittherapy.com/download%20center/artilces/Strenght%20Training%20and%20CP.pdf.
20.
Liaqat S, Butt MS, Javaid HMW. Effects of Universal Exercise Unit Therapy on Sitting Balance
in Children with Spastic and Athetoid Cerebral Palsy: A Quasi-Experimental Study. Khyber Medical
N A P A T h e r a p y R e s e a r c h
P a g e |
31
Page 60 of 153

FOI 24/25-0473
University Journal [Internet]. 2016; 8(4):[177- pp.]. Available from:
http://www.kmuj.kmu.edu.pk/article/view/16786.
21.
Emara HA, El-Gohary TM, Al-Johany AA. Effect of body-weight suspension training versus
treadmill training on gross motor abilities of children with spastic diplegic cerebral palsy. Eur J Phys
Rehabil Med [Internet]. 2016 Jun; 52(3):[356-63 pp.]. Available from:
https://pubmed.ncbi.nlm.nih.gov/26845668/.
22.
Menz SM, Hatten K, Grant-Beuttler M. Strength Training for a Child With Suspected
Developmental Coordination Disorder. Pediatric Physical Therapy [Internet]. 2013; 25(2). Available
from:
https://journals.lww.com/pedpt/Ful text/2013/25020/Strength Training for a Child With Suspect
ed.18.aspx.
23.
Bar-Haim S, Harries N, Belokopytov M, Frank A, Copeliovitch L, Kaplanski J, et al. Comparison
of efficacy of Adeli suit and neurodevelopmental treatments in children with cerebral palsy.
Developmental Medicine & Child Neurology [Internet]. 2006; 48(5):[325-30 pp.]. Available from:
https://onlinelibrary.wiley.com/doi/abs/10.1017/S0012162206000727.
24.
Nemkova SA, Kobrin VI, Sologubov EG, Iavorskiĭ AB, Sinel'nikova AN. [Regulation of vertical
posture in patients with children's cerebral paralysis treated with the method of proprioceptive
correction]. Aviakosm Ekolog Med [Internet]. 2000 2000; 34(6):[40-6 pp.]. Available from:
http://europepmc.org/abstract/MED/11253723.
25.
Gracies J-M, Marosszeky JE, Renton R, Sandanam J, Gandevia SC, Burke D. Short-term effects
of dynamic Lycra splints on upper limb in hemiplegic patients. Archives of Physical Medicine and
Rehabilitation [Internet]. 2000 2000/12/01/; 81(12):[1547-55 pp.]. Available from:
http://www.sciencedirect.com/science/article/pi /S0003999300546231.
26.
Morris C, Bowers R, Ross K, Stevens P, Phillips D. Orthotic management of cerebral palsy:
recommendations from a consensus conference. NeuroRehabilitation [Internet]. 2011; 28(1):[37-46
pp.]. Available from: https://strathprints.strath.ac.uk/40443/1/ful text.pdf.
27.
Bailes AF, Greve K, Schmitt LC. Changes in Two Children with Cerebral Palsy After Intensive
Suit Therapy: A Case Report. Pediatric Physical Therapy [Internet]. 2010; 22(1). Available from:
https://journals.lww.com/pedpt/Ful text/2010/02210/Changes in Two Children with Cerebral Pa
lsy After.11.aspx.
28.
Neves EB, Krueger E, de Pol S, de Oliveira MCN, Szinke AF, de Oliveira Rosário M. Benefits of
intensive neuromotor therapy (TNMI) for the control of the trunk of children with cerebral palsy.
Neuroscience Magazine [Internet]. 2013; 21(4):[549-55 pp.]. Available from:
https://periodicos.unifesp.br/index.php/neurociencias/article/download/8141/5673.
29.
Datorre E. Intensive Therapy Combined with Strengthening Exercises Using the Thera Suit in
a child with CP: A Case Report. American Association of Intensive Pediatric Physical Therapy
[Internet]. 2005. Available from:
http://www.suittherapy.com/pdf%20research/Int.%20Therapy%20%20Research%20Datore.pdf.
30.
Semenova KA. Basis for a method of dynamic proprioceptive correction in the restorative
treatment of patients with residual-stage infantile cerebral palsy. Neuroscience and Behavioral
Physiology [Internet]. 1997 1997/11/01; 27(6):[639-43 pp.]. Available from:
https://doi.org/10.1007/BF02461920.
31.
NAPA Centre. NeuroSuit 2020 [Available from: https://napacentre.com.au/our-
programs/neurosuit/.
32.
Ability Plus Therapy. Intensive suit therapy 2014 [Available from:
http://abilityplustherapy.com/got-therapy/intensive-suit-therapy/.
N A P A T h e r a p y R e s e a r c h
P a g e |
32
Page 61 of 153

FOI 24/25-0473
33.
Karadağ-Saygı E, Giray E. The clinical aspects and effectiveness of suit therapies for cerebral
palsy: A systematic review. Turk J Phys Med Rehabil [Internet]. 2019; 65(1):[93-110 pp.]. Available
from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6648185/.
34.
Centre N. Cuevas Medek Exercise 2020 [Available from: https://napacentre.com.au/our-
programs/intensive-therapy/.
35.
Vanderminden JA. The social construction of disability and the modern-day healer 2009.
36.
de Oliveira GR, Fabris Vidal M. A normal motor development in congenital hydrocephalus
after Cuevas Medek Exercises as early intervention: A case report. Clinical Case Reports [Internet].
2020; 8(7):[1226-9 pp.]. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/ccr3.2860.
37.
Mitroi S. Stimulation of triple extension tone and orthostatic balance in the child with
cerebral palsy through exercises specific to medek method. PhysicalEducation, Sport and
KinesiologyJournal [Internet]. 2016; 1(43):[48-51 pp.]. Available from:
https://discobolulunefs.ro/Reviste/2016/Discobolul ful paper 43 1 2016 v2.pdf#page=47.
38.
Tanner K, Schmidt E, Martin K, Bassi M. Interventions Within the Scope of Occupational
Therapy Practice to Improve Motor Performance for Children Ages 0–5 Years: A Systematic Review.
American Journal of Occupational Therapy [Internet]. 2020; 74(2):[7402180060p1-p40 pp.].
Available from: https://doi.org/10.5014/ajot.2020.039644.
39.
Longo E, de Campos AC, Palisano RJ. Let's make pediatric physical therapy a true evidence-
based field! Can we count on you? Brazilian journal of physical therapy [Internet]. 2019 May-Jun;
23(3):[187-8 pp.]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6531638/.
40.
Sakzewski L, Ziviani J, Boyd RN. Efficacy of Upper Limb Therapies for Unilateral Cerebral
Palsy: A Meta-analysis. Pediatrics [Internet]. 2014; 133(1):[e175-e204 pp.]. Available from:
https://pediatrics.aappublications.org/content/pediatrics/133/1/e175.full.pdf.
41.
Elgawish M, Zakaria M. The effectiveness of intensive versus standard physical therapy for
motor progress in children with spastic cerebral palsy. Egyptian Rheumatology and Rehabilitation
[Internet]. 2015 January 1, 2015; 42(1):[1-6 pp.]. Available from:
http://www.err.eg.net/article.asp?issn=1110-
161X;year=2015;volume=42;issue=1;spage=1;epage=6;aulast=Elgawish.
42.
Novak I, Cusick A, Lannin N. Occupational Therapy Home Programs for Cerebral Palsy:
Double-Blind, Randomized, Controlled Trial. Pediatrics [Internet]. 2009; 124(4):[e606-e14 pp.].
Available from: https://pediatrics.aappublications.org/content/pediatrics/124/4/e606.full.pdf.
43.
Lin K-c, Wang T-n, Wu C-y, Chen C-l, Chang K-c, Lin Y-c, et al. Effects of home-based
constraint-induced therapy versus dose-matched control intervention on functional outcomes and
caregiver well-being in children with cerebral palsy. Research in Developmental Disabilities
[Internet]. 2011 2011/09/01/; 32(5):[1483-91 pp.]. Available from:
http://www.sciencedirect.com/science/article/pi /S0891422211000242.
44.
Eliasson A-C, Shaw K, Berg E, Krumlinde-Sundholm L. An ecological approach of Constraint
Induced Movement Therapy for 2–3-year-old children: A randomized control trial. Research in
Developmental Disabilities [Internet]. 2011 2011/11/01/; 32(6):[2820-8 pp.]. Available from:
http://www.sciencedirect.com/science/article/pi /S089142221100196X.
45.
Hoare B, Imms C, Villanueva E, Rawicki HB, Matyas T, Carey L. Intensive therapy following
upper limb botulinum toxin A injection in young children with unilateral cerebral palsy: a randomized
trial. Developmental Medicine & Child Neurology [Internet]. 2013; 55(3):[238-47 pp.]. Available
from: https://onlinelibrary.wiley.com/doi/abs/10.1111/dmcn.12054.
N A P A T h e r a p y R e s e a r c h
P a g e |
33
Page 62 of 153

FOI 24/25-0473
46.
Rosenbaum PL, Walter SD, Hanna SE, Palisano RJ, Russell DJ, Raina P, et al. Prognosis for
Gross Motor Function in Cerebral PalsyCreation of Motor Development Curves. JAMA [Internet].
2002; 288(11):[1357-63 pp.]. Available from: https://doi.org/10.1001/jama.288.11.1357.
47.
Holmefur M, Krumlinde-Sundhold L, Bergstrom J, Eliasson A-C. Longitudinal development of
hand function in children with unilateral cerebral palsy. Developmental Medicine & Child Neurology
[Internet]. 2010; 52(4):[352-7 pp.]. Available from:
https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1469-8749.2009.03364.x.
48.
Haley SM. Pediatric Evaluation of Disability Inventory (PEDI): Development, standardization
and administration manual: Therapy Skill Builders; 1992.
49.
Palisano RJ, Hanna SE, Rosenbaum PL, Russell DJ, Walter SD, Wood EP, et al. Validation of a
Model of Gross Motor Function for Children With Cerebral Palsy. Physical Therapy [Internet]. 2000;
80(10):[974-85 pp.]. Available from: https://doi.org/10.1093/ptj/80.10.974.
50.
Hanna SE, Bartlett DJ, Rivard LM, Russell DJ. Reference Curves for the Gross Motor Function
Measure: Percentiles for Clinical Description and Tracking Over Time Among Children With Cerebral
Palsy. Physical Therapy [Internet]. 2008; 88(5):[596-607 pp.]. Available from:
https://doi.org/10.2522/ptj.20070314.
51.
Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and
management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. The
Lancet Neurology [Internet]. 2010 2010/02/01/; 9(2):[177-89 pp.]. Available from:
http://www.sciencedirect.com/science/article/pi /S1474442209702728.
52.
Lurio JG, Peay HL, Mathews KD. Recognition and management of motor delay and muscle
weakness in children. Am Fam Physician [Internet]. 2015 Jan 1; 91(1):[38-44 pp.]. Available from:
https://www.aafp.org/afp/2015/0101/p38.html.
53.
Pascual JM. Progressive Brain Disorders in Childhood. Cambridge: Cambridge University
Press; 2017.
54.
Jan MM, Shaabat AO. Clobazam for the treatment of intractable childhood epilepsy.
Neurosciences (Riyadh) [Internet]. 2000 Jul; 5(3):[159-61 pp.]. Available from:
http://www.nsj.org.sa/pdffiles/Jul00/Clobazam.pdf.
55.
Jan MM. Clinical approach to children with suspected neurodegenerative disorders.
Neurosciences [Internet]. 2002; 7(1):[2-6 pp.]. Available from:
https://www.researchgate.net/profile/Mohammed Jan/publication/227859386 Clinical approach
to children with suspected neurodegenerative disorders/links/09e414fe6f11164c36000000.pdf.
56.
Olney RS, Doernberg NS, Yeargin-Allsop M. Exclusion of progressive brain disorders of
childhood for a cerebral palsy monitoring system: a public health perspective. J Registry Manag
[Internet]. 2014 Winter; 41(4):[182-9 pp.]. Available from:
https://pubmed.ncbi.nlm.nih.gov/25803631.
57.
Christiansen AS, Lange C. Intermittent versus continuous physiotherapy in children with
cerebral palsy. Developmental Medicine & Child Neurology [Internet]. 2008; 50(4):[290-3 pp.].
Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1469-8749.2008.02036.x.
58.
Bower E, Michell D, Burnett M, Campbell MJ, McLellan DL. Randomized controlled trial of
physiotherapy in 56 children with cerebral palsy followed for 18 months. Developmental Medicine &
Child Neurology [Internet]. 2001; 43(1):[4-15 pp.]. Available from:
https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1469-8749.2001.tb00378.x.
59.
Almeida KM, Fonseca ST, Figueiredo PRP, Aquino AA, Mancini MC. Effects of interventions
with therapeutic suits (clothing) on impairments and functional limitations of children with cerebral
N A P A T h e r a p y R e s e a r c h
P a g e |
34
Page 63 of 153

FOI 24/25-0473
palsy: a systematic review. Brazilian Journal of Physical Therapy [Internet]. 2017 2017/09/01/;
21(5):[307-20 pp.]. Available from:
http://www.sciencedirect.com/science/article/pi /S1413355517302484.
N A P A T h e r a p y R e s e a r c h
P a g e |
35
Page 64 of 153










































