


FOI 5328 IR - Document 1
General use items
Pre-consultation paper – 27 June 2024
workshop with private health insurance
stakeholders
under Care
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the the
by
Page 1 of 3
FOI 5328 IR - Document 1
Overview
On 1 May 2024, the Minister of Health and Aged Care announced that general use items (GUI)
would be retained in Part D of the Prescribed List (PL). This decision follows continuous feedback
from multiple stakeholders that removing the GUIs from the PL would have negative clinical
implications and potential adverse outcomes for patients.
The announcement comes two years after the initial planned removal of the GUIs from the PL,
and a year after insurers and hospitals were requested to negotiate alternative funding
arrangements.
We acknowledge the concerns of private health insurers that the announcement about retaining
GUI on the PL represent. The department is undertaking further consultation and engagement to
identify ways in which these concerns might be addressed – both regulatory and non-regulatory.
What we invite you to do
We ask that you provide us with practical suggestions about ways to increase the integrity of the
settings of the PL as well as mechanisms to reduce increased growth in usage of GUIs per
episode of care and the resulting increased growth in expenditure (i.e. without any clinical need).
under
In considering your input to this matter we ask that you provide as much detail and eviden
Care
ce as
possible. Please ensure your suggestions remain in the context of the Prescribed List and are
reasonable, pragmatic and within the authority of the department.
1982 Aged
Questions
released
Act and
At the workshop, we would like to discuss your answers to the following questions.
been
Integrity
has
Health
of
1.
What do you see are the key areas of concern for the integrit
Information
y of the PL settings in the
context of the GUIs?
of
2.
If you were to consider prioritisation of these, what would that look like?
document
3.
What are the potential system based-actions (i.e. not fixing of individual errors) that could
be taken, by who and when?
This Freedom
Department
4.
How would you suggest the success of these actions are measured?
the the
5.
What are the likely consequences – positive/negative and who would they effect?
by
Utilisation and growth in expenditure
6. What sub-categories of GUIs on the PL represent the key areas of growth in utilisation per
episode of care and therefore increase in benefit expenditure?
7. Are there specific procedures that represent higher growth in utilisation?
8. If there are concerns that the growth in use is not related to clinical need, how is this
determined/measured? Who can validate this?
9. What system-based mechanisms are either in place or need to be put in place to address
this problem?
a. Would these mechanisms be different if there was a demonstrated clinical need?
10. How would you suggest the success of these actions are measured?
11. What are the likely consequences – positive/negative and who would they effect?
General use items - Pre-consultation paper
3
Page 2 of 3
FOI 5328 IR - Document 1
Other matters
12. Are there other areas of concern with the retention of GUIs on the PL that need to be
considered?
under Care
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the the
by
Page 3 of 3

under Care
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the the
by
under Care
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the the
by

FOI 5328 IR - Document 4
General Use items controls and tweaks to the list
DRAFT AS AT NOON THU 2 MAY 2024
PHA’s major concern is volume and cost being added with no demonstrable clinical
benefits. For example, there was a 12.9% volume growth in GUI in 2017/18 on flat
surgery volumes.
Recommended overall rules
• Price/volume agreements
o If the total use of items under each subcategory increases by more than
10% in any year (adjusted for any increases in surgical volume), the price
of al items under that code should be reduced by 10% (rounded up to the
nearest dollar).
• Ensure no out of pocket costs for consumers as a conditi
under on of listing. This is
Care
likely to require the price to hospitals not to exceed the PL price.
• Any price increases (i.e. through amendment application
1982 s) to
Aged demonstrate a
public interest case, including the clinical and ec
Act o
released nomic benefits. These public
and
interest cases should be published by the Minister each PL cycle.
• Hospitals provide feedback on the costs
been of medical devices and standard usage
patterns to their medical staff (as previously offer
Health ed by hospital groups).
has
• Where hospitals use general use items at a
of si
Information gnificantly higher rate than their
peer group (for example, over 20
of % to 50% higher than the average depending on
distribution), payment wil be provided in ful only where the treating doctors
certify that the unusual use
document is reasonable and necessary, otherwise a 120% to
150% expenditure cap wil apply.
Freedom
Department
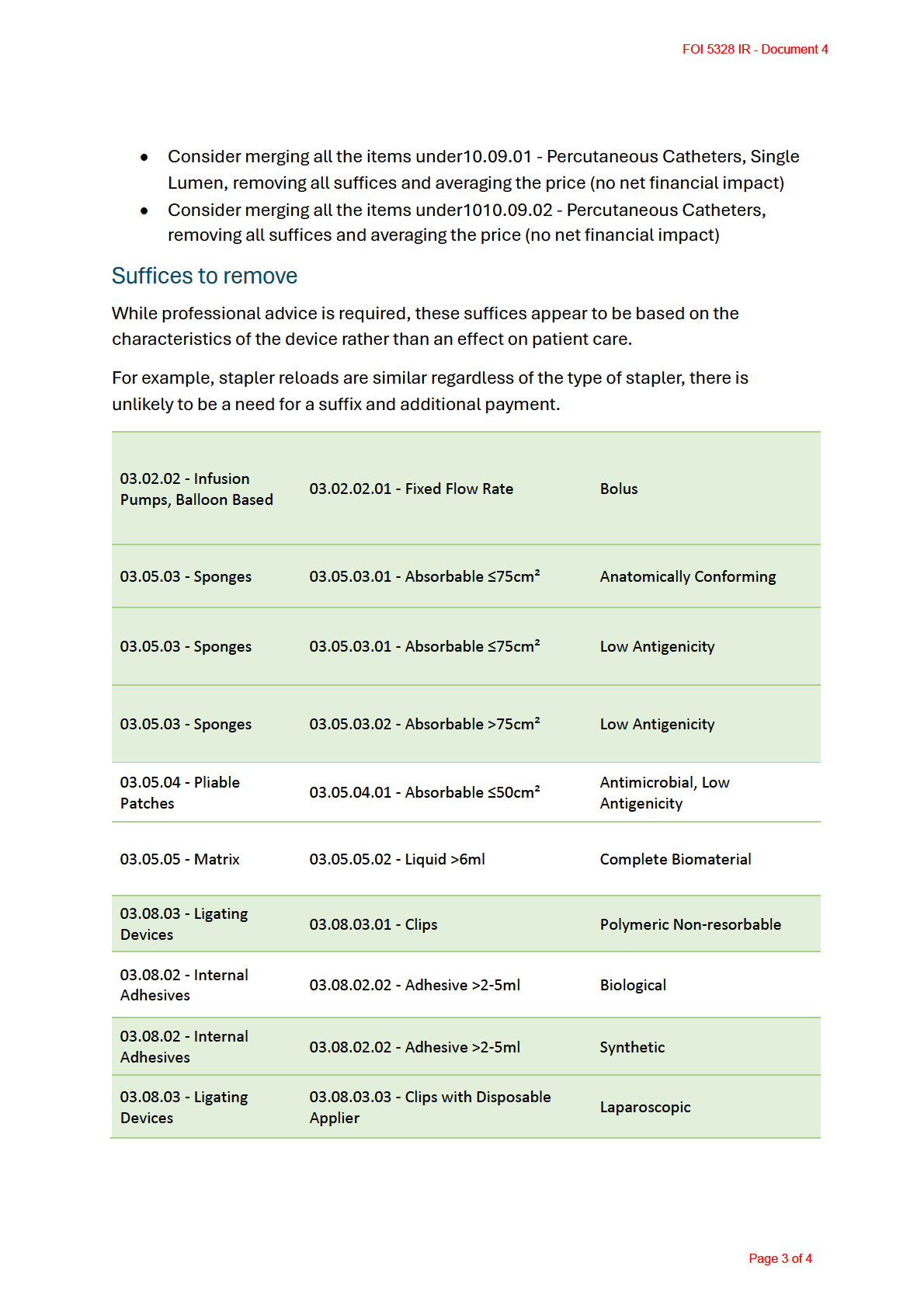
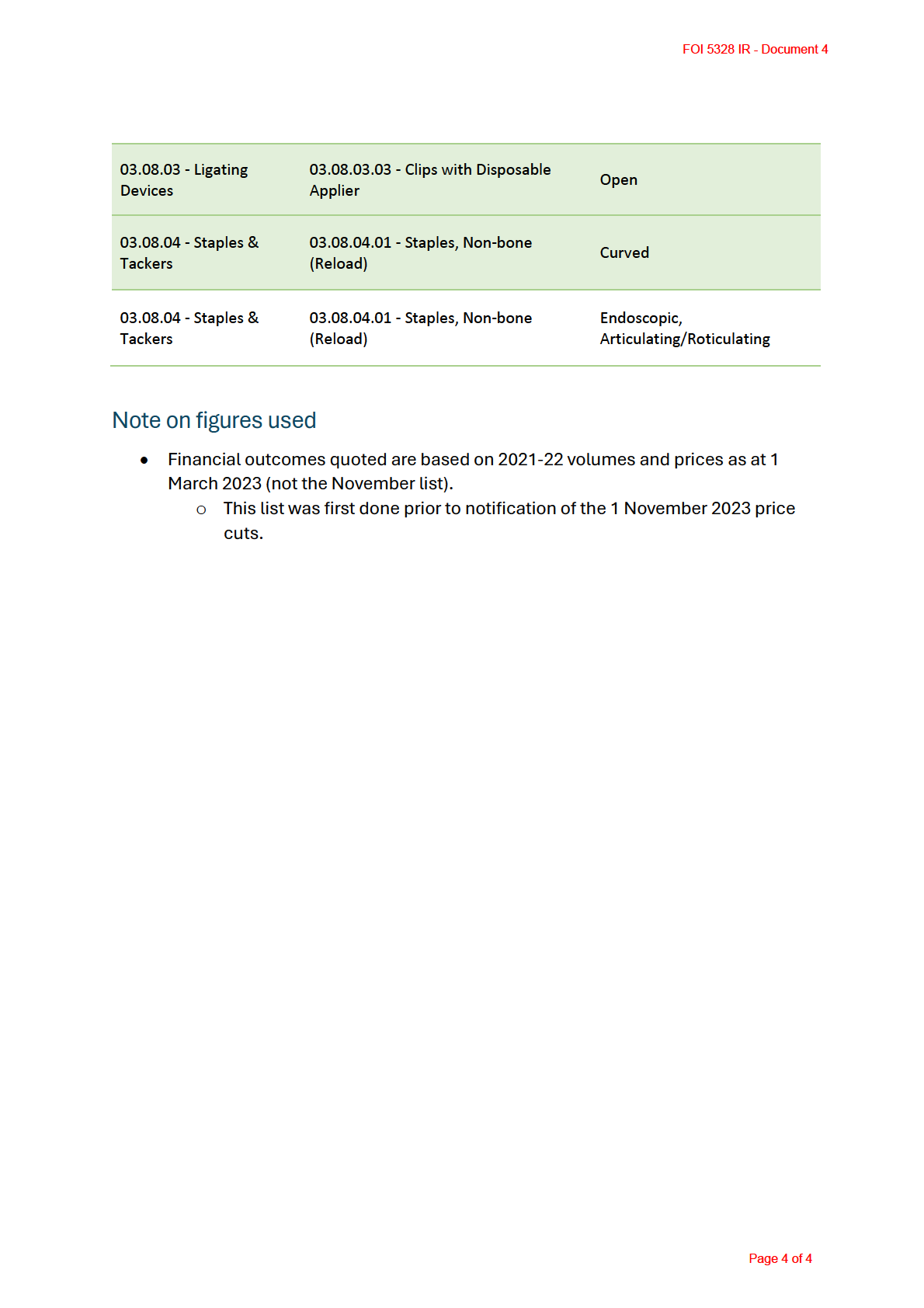
• Remove suffices
This which do not impact on the patient outcome. These would
the the
include price reductions where there is no consumer benefit from device
by
characteristics (we have a list to consider, see below)
Fix mistakes on the list
• Address error in pricing and al adhesion barriers with the same ARTG back to
public price (reduce spend by $1.018m)
• Use the Surgiflo price 6ml for Floseal, Purastat 5mls as there’s no difference in
price at the higher volume between Floseal and Surgiflo (reducing spend by
$0.620m)
• Hemoblast VB002 reduced to same price as Floseal, Surgiflo (reducing spend by
$0.225m)
• Applicators (03.05.05.05 - Accessory Extender) removed, as they should be
incorporated into the device as per public prices (reducing spend by $0.022m)
Page 1 of 4
FOI 5328 IR - Document 4
• Move ET082 PureRegen Gel Sinus from adhesion barriers to nasal code (no price
impact)
• Remove internal adhesive applicators (03.08.02.04 - Adhesive Accessory) as
they should be included in device cost as per public prices (reduce spend by
$1.792m)
• Add conditions of use for Tisseal etc to vascular and dura consistent with IFU
• Remove Evicel as it is a listed medicine, not a device (not eligible)
• Remove ET065 as it is a suture and not eligible
• Remove ET066 as not eligible (reduce spend by $1.081m)
• Tristapler MI287 and GIA stapler AS209 repriced to the sum of the component
parts (reduce spend by $2.004m)
• Remove CoreKnot, these are surgical instruments (DE606, DE609)
• Remove anomaly where larger sponges receive much higher remuneration,
change to per cm for al sizes (reduce spend by $0.083m)
• Reprice all liquid repair sealants to the highest volume price, rather than paying
under
more for the smal er sizes.
Care
• Place condition on use for al liquid repair sealants to dura, as per IFUs
1982
• ER279 OverStitchTM Endoscopic Suturing System repriced to
Aged comparator FQ002
released
Act
Recommended price reviews
and
been
• Remove premium for powered stapler as no HTA assessment was undertaken
(reduce spend by $1.129m)
has
Health
of
• Remove premium for endoscopic suffice f
Information or staplers as no clear difference in
performance in most instanc
of es (reduce spend by $12.759m).
• Reprice KI010 to $90 as it is readily available at that price (here)
document
• Reduce price for 03.08.04.04 - Staplers, Non-bone with Disposable Applier to
the same as 03.08.04.02
Freedom – Staplers
Department . There is no justification for the premium.
This
Suggested changes t
the o impr
the ove integrity
by
• Remove capital items for infusion pumps and increase cassette cost to
compensate (no net financial impact).
o Remove 03.02.03 - Infusion Pumps, Battery Powered
o The price of the 03.02.05.02 - Administration Cassettes would need to
increase from $26 to $51 to compensate for these items coming off
• Use a single price for pliable patches to remove incentives for larger sizes (no net
financial impact)
• Use a per gram price for haemostatic power to remove incentives for larger sizes
(no net financial impact)
• Use a single price for absorbable sponges to remove incentives for larger sizes
(no net financial impact)
Page 2 of 4
under Care
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the the
by
under Care
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the the
by
under Care
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the the
by
under Care
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the the
by
under Care
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the the
by
FOI 5328 IR - Document 5
Subject: RE: Meeting/workshop request on general use items [SEC=OFFICIAL]
Thanks Andrew
Ben Harris
DIRECTOR OF POLICY AND RESEARCH
M: s47F
|
E: s47F
@pha.org.au
www.privatehealthcareaustralia.org.au
<image002.jpg>
Follow us on
<image002.jpg>
From: RINTOUL, Andrew <s22
@health.gov.au>
Sent: Thursday, May 9, 2024 4:35 PM
To: Ben Harris <s47F
@pha.org.au>
Cc: s22
health.gov.au>; s22
@Health.gov.au>; s22
@health.gov.au>; s47F
s47F
; Rachel David <s47F
@pha.org.au>;
s22
@Health.gov.au>
Subject: RE: Meeting/workshop request on general use items [SEC=OFFICIAL]
under Care
Hi Ben,
I’ll ask s22
to coordinate internally and come back to you with a range of
1982
times to hold the meeting.
Aged
Kind regards
released
Act and
Andrew
Andrew Rintoul
been
Assistant Secretary
<image003.png>
has
Health
Information
of
Protheses List Reform Taskforce | Technology Assessment and Access Division
of
Australian Government Department of Health and Aged Care
T: +61 2 6289 s22 | M: s22
E: s22
@health.gov.au
Location: Sirius 9.N.101document
PO Box 9848, Canberra ACT 2601, Australia
The Department of Health acknowledges the traditional owners of country throughout Australia, and
This Freedom
Department
their continuing connection to land, sea and community. We pay our respects to them and their cultures,
and to elders both past and present.
the the
From: Ben Harris <s47F
by
@pha.org.au>
Sent: Wednesday, May 8, 2024 5:12 PM
To: RINTOUL, Andrew <s22
@health.gov.au>
Cc: s22
@health.gov.au>;s22
@Health.gov.au>; s22
@health.gov.au>; s47F
s47F
; Rachel David <s47F
@pha.org.au>
Subject: Meeting/workshop request on general use items
REMINDER: Think before you click! This email originated from outside our organisation. Only
click links or open attachments if you recognise the sender and know the content is safe.
Andrew,
Rachel and I met with s47F
and s47F
on Friday on general use items
on the PL. They have encouraged us to engage with you on our list of errors,
integrity issues and consumer protection measures sent last week.
I ask for an extended meeting/workshop with you and your staff on the 40 issues
Page 4 of 5
FOI 5328 IR - Document 5
we have raised with general use items. I recognise that other than s22 many of
your staff have not been around long enough to have had exposure to the general
use item history – in particular, the EY report and the department’s report on
general use items. We have the advantage of the history and the data from funds
to add to the repository of knowledge the department has collected over the years,
plus the expertise of former device company staff who will be able to help the
department come to decisions on how to proceed with protecting consumers’
interests.
We propose going through the technical suggestions, where they have come from
(eg the EY report), and why we are recommending what we are recommending (eg
using the Hereco framework for regrouping). Our line-by-line examination of the
general use category as part of the investment we have made while looking for a
solution to general use items should be of value to the taskforce.
I think we could get it done in three hours, with me, s47F and s47Fgoing through
the list of recommendations to inform your decisions going forward.
Let me know when would suit you and your team,
Thanks
Ben
Ben Harris
under
DIRECTOR OF POLICY AND RESEARCH
Care
M: s47F
|
E: s47F
@pha.org.au
www.privatehealthcareaustralia.org.au
1982 Aged
<image002.jpg>
released
Act and
Follow us on
<image002.jpg>
been Health
"Important: This transmission is intended only for the use of the
has Information
of
addressee and may contain confidential or legally privileged information.
of
If you are not the intended recipient, you are notified that any use or
dissemination of this communication is strictly prohibited. If you receive
document
this transmission in error please notify the author immediately and delete
Freedom
Department
all copies of this transmission."
This
the the
"Important: This transmission is intended only for the use of the
by
addressee and may contain confidential or legally privileged information.
If you are not the intended recipient, you are notified that any use or
dissemination of this communication is strictly prohibited. If you receive
this transmission in error please notify the author immediately and delete
all copies of this transmission."
Page 5 of 5
under Care
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the the
by
FOI 5328 IR - Document 6
s22
under Care
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the the
by
Page 2 of 4
FOI 5328 IR - Document 6
s22
under Care
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the the
by
Page 3 of 4
FOI 5328 IR - Document 6
s22
under Care
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the the
by
Page 4 of 4
under Care
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the the
by

FOI 5328 IR - Document 8
Prescribed List Reforms - Consultation Paper 8a:
Gifts, benefits and discounts reporting requirements
under Care
1982 Aged
June 2024
released
Act and
been
has
Health
Information
of
of
document
Freedom
Department
Contact:
This
the the
Ben Harris, Director Policy an
by d Research
s47F
@pha.org.au
Page 1 of 8
FOI 5328 IR - Document 8
About Private Healthcare Australia (PHA)
Private Healthcare Australia (PHA) is the Australian private health insurance industry’s peak
representative body. We have 22 registered health funds throughout Australia as members and
col ectively represent 98% of people covered by private health insurance. PHA member funds
provide healthcare benefits for 14.4 million Australians.
Introduction
PHA welcomes the opportunity to contribute to the Department of Health and Aged Care’s
consideration of improving compliance and transparency for medical devices. However, the
proposals outlined in this discussion paper are clearly insufficient. While they may help improve the
current situation, these proposals fall well short of current integrity and transparency standards.
PHA recommends medical devices be regulated in the same manner as pharmaceuticals, with
mandatory reporting of all gifts, benefits or discounts to a public registry as part of an enforceable
code of conduct that aligns with the code of conduct for pharmaceutical companies. This must
include penalties for compliance breaches if it is to be effective at any level.
There is no public policy justification for an integrity framework for medical devices which is less
rigorous than that for pharmaceuticals.
under Care
The current market for medical devices is beset by graft, gifts and various financial incentives with
1982
little to no transparency to consumers, payors and the general public. Where m
Aged oney is changing
hands without the benefits of transparency, market manipulation
released
Act and influencing clinical decisions is
much more likely to occur, and the spectre of corruption looms over th
and e sector.
The losers are consumers – patients who may be recei
been ving less optimal, or even harmful, medical
devices, and those paying for health care (consumers, taxpayers a
Health nd payors, including health funds).
has Information
of
Tentative steps are not enough – allowing a little bit of corruption is not acceptable to health funds.
of
The Australian Government should do al it can to ensure consumers are receiving the best possible
medical devices, which can only occur where there is full transparency over all types of payments
and gifts to providers.
document
Freedom
Department
Gifts, benefits and
This discounts reporting requirements
the the
There are multiple ways in which sponsors and hospitals co-operate to provide discounts, gifts, free
by
products, cash rebates and ‘transfers of value arrangements’ to encourage use of Prescribed List
items in the private sector.
Page 2 of 8
FOI 5328 IR - Document 8
These practices:
- Reduce competition in the market because some smal er companies do not have the
resources to use them.
- Keep PL rebates high because there is no transparency of true market value.
- Encourage wasteful practices such as unnecessary use of PL items.
- Drive low value care that can harm consumers and the broader health system.
- Drive the provision of poorly performing devices when better options are available.
Rather than the discount being provided to the ultimate payer of the goods, i.e. the holders of
private health insurance (PHI) and the taxpayers who subsidise PHI, they are banked by the hospital
owners who act as intermediaries without risk in the process.
These practices are not restricted to PL items, but PL items are often involved due to the known high
minimum rebate prices. There are several ways in which the set rebate price for PL items is used by
sponsors and hospitals for this purpose. This includes:
Cash rebates
This is a very common mechanism used by hospitals to elicit a discount on PL items. A hospital group
typically approaches a sponsor to inform them that they will only allow their products to be used in
under
their hospitals if a certain percentage of the PL rebate is paid back to the hospital ow
Care ner in the form
of a cash rebate. This practice has been happening since the rules for the PL changed in the late
1990s/ early 2000s, so that PHI is only obliged to pay the invoiced amoun
1982 t.
Aged
released
Act
The rebates can be extended to include other non-PL items from the supplier utilised in the hospital,
and
e.g. disposable items such as power saw blades and orthopaedic space suits. This provides a
competitive advantage to larger multinational compa
been nies that can bundle a wide range of PL and
non-PL items. Smaller local sponsors who may specialise in a parti
Health cular surgical specialty or who do
has
not have the capacity to supply such a wide range of ite
of ms struggle to compete with this.
Information
These opaque rebate arrangements have led
of to the significant increase in PL items per case over
recent years. This can be seen with the significant increase in general use items (GUI) being used in
certain surgeries, such as Evicel in hip rep
document lacements, often without any evidence or a comparable
increase in the same usage in the public system. Evicel sales in hip replacements are almost
Freedom
Department
exclusively limited to where a
This J&J (Evicel) representative attended the case. This indicates it is not a
routine item in use for that su
the rgical i
the ntervention unless a sales representative attends for the device
company.
by
Global price fixing
Private hospital providers that have a multinational presence can negotiate with multinational
medical device companies to fix a global price for the PL items used in their Australian facilities. This
al ows the hospital operator to benefit from the fixed rebate price of the PL in two ways. Firstly, they
Page 3 of 8
FOI 5328 IR - Document 8
can take advantage of the revenue generated from the difference in the two prices and secondly,
they can offshore that difference to a low tax jurisdiction. This practice sees consumers and taxpayers
lose out in two ways from not receiving the discounted price through lower premiums, and from the
loss of tax revenue for the federal government which subsidises PHI premiums.
Company dollars schemes
This is a mechanism used by larger multinational companies to ensure their PL items are used within
a hospital. This mechanism works on a similar principle to the cash rebates in that a hospital will earn
company dol ars or credits that can later be redeemed to purchase other non-PL items such as
capital equipment or disposables (e.g. Stryker Dollars, Zimmer Dollars or Medtronic Dollars). This
effectively provides the hospital with a discount as non-PL goods are provided free of charge through
the use of PL items. This again gives an unfair competitive advantage to larger multinational
companies.
Free capital
Another common practice is the offer of high-cost capital equipment that is linked directly to the use
of PL items, e.g. the placement of an orthopaedic surgical assist device where the contract states a
specific number of joint replacement devices must be used from that company per year over a
specific number of years within the hospital. These deals can lead to devices being used that do not
under
provide the patient with the best outcome because the surgeon is encouraged to us
Caree the device that
will pay for the capital equipment.
1982 Aged
Straight discounts
released
Act
Despite the change in rules for the PL mentioned above, PHA has been made aware of surgeon
and
owned day surgery facilities being given a direct discount for PL devices that are then charged to PHI
at the ful rebate amount. The surgeon owned day su
been rgery then simply banks the difference as profit
at the expense of consumers and taxpayers. This sort of behaviou
Health r encourages surgeons who own
has
the facility to implant devices that make them the most p
of rofit rather than what wil provide the best
Information
outcome for the patient.
of
Fellowships
The medical device industry has a history
document of sponsoring specific surgical fellowships to promote their
devices. However, these fellowships are usual y tied, not just to a surgical practice, but also to a
Freedom
Department
specific hospital. This is ofte
This n a public hospital, but private hospitals have also been involved. There is
an implied obligation for the s
the urgeon
the s and hospitals involved in these fellowship arrangements to
utilise the sponsor company’s P
by L devices. Whilst many of these fellowships offer a valuable expert
training experience that improves the skil s and knowledge of those who are selected to undertake
them, they are yet another way that the high rebates mandated on the PL can be utilised by hospitals
to, in effect, receive a free or greatly discounted surgeon funded by consumers and taxpayers.
Page 4 of 8
FOI 5328 IR - Document 8
Free samples
Whilst the offer of free samples for a trial period is common commercial practice across most
industries as a competitive tool to generate new business and clients, as the consultation paper
states, “the PL is a regulated reimbursement mechanism that does not follow standard commercial
arrangements and any incorrect or inappropriate claiming for items against the PL ultimately affects
the insured patient.” The hospital involved in this activity is in effect receiving 100% of the PL rebate
amount as revenue rather than the end consumer and taxpayer. Any PL items offered free of charge,
for whatever reason, should not then be bil ed through to PHI. This would al ow sponsors and
hospitals to engage in a normal commercial practice whilst still being required to report the
transaction on the registry.
Public hospitals
All public hospitals in Australia purchase their surgical devices via a public tender process. The
majority of these are done as panel tenders, i.e. al companies are accepted onto the tender. This is
done to drive down the prices paid by state governments and to allow surgeons a choice of devices.
As this is a competitive process, suppliers routinely offer their devices at a discount to the rebates on
the PL. However, despite purchasing the devices at the discounted rate, PHA has evidence that public
hospitals stil invoice the PHI of any patient that receives treatments as a private patient the ful PL
rebate amount, thus using the PL system to generate funding. The public h
under ospital and, by default, the
Care
state governments are therefore using PHI members and federal taxpayers to enhance their budget
positions.
1982 Aged
Marketing assistance incentives
released
Act
Like fellowships, surgeons and hospitals are offered a range of marketi
and ng assistance as incentives by
the medical device companies to use their devices. This can be as simple as providing generic patient
been
brochures and videos explaining the device and procedure to more bespoke / individualised glossy
Health
patient information packs. Whilst this may seem
has to be standard marketing activity, it is being funded
of
by PHI members and the taxpayer via PL rebates. Any direct t
Information o consumer promotion of medical
devices should be completely banned. of
The majority of these types of discount / incentives and transfer of value arrangements are well
document
known across the healthcare industry but have always been kept opaque by the parties involved,
often hidden behind ‘commercial in confi
Freedom dence’ agre
Department ements between device companies and
This
hospitals and medical practitioners. However, as can be seen in the Senate Committee report from
the the
2016, the department has known about them for some time.
by
Recommendations
Mandatory reporting of gifts, benefits or discounts to a public registry.
There should be mandatory reporting of al discounts / incentives and ‘transfer of value
arrangements’ between sponsors, hospitals and medical practitioners to a registry that is published
Page 5 of 8
FOI 5328 IR - Document 8
quarterly each year. Sponsors and hospitals should be required to report al such transactions, and
other people with knowledge of these transactions should be permitted to report them to prevent
under reporting. The reporting should detail al individual PL items involved as well as the total value
of the incentives provided to hospitals and medical practitioners. There should be penalties for non-
compliance.
There should be no threshold – all transfers of value must be reported to ensure integrity.
Medical device companies should be responsible for populating the public registry, but those
receiving the benefits should bear responsibility for ensuring it is done correctly. In effect, this would
require large penalties for device companies who fail to disclose, and public warnings and smaller
penalties for hospitals and medical practitioners to encourage them to ensure the benefits received
are properly disclosed by the device companies.
The current opacity of transactions between medical device companies and providers al ows for
gaming of the PL system, creating unnecessary additional costs that ultimately flow through to
consumers during a cost-of-living crisis. A public registry will help reveal the true value of PL items,
which should then be reflected in the PL rebate amount, currently set by the Australian Government.
Public reporting of these activities will bring transparency for all involved, particularly consumers
who pay for private health insurance to contribute to the cost of their ow
under n healthcare.
Care
An enforceable code of conduct for medical devices that aligns with the code of conduct for
1982
pharmaceutical companies.
Aged
Like the pharmaceutical industry, the medical device industry is a
released
Act multi-billion-dollar sector in
Australia. Medical technology companies engage in many activities to b
and uild relationships with health
professionals and promote sales of their products, including:
been
- company-sponsored educational events;
has
Health
- providing cash payments to medical specialists t
of o participate in product development or
Information
advisory boards
of
- engaging key opinion leaders as speakers or consultants;
- paying for travel, meals or professional development; and
document
- sponsoring post-market trials.
Freedom
Department
PHA wants an enforceable
This Code of Conduct for the medical technology industry that aligns with the
code of conduct for pharmac
the eutical c
the ompanies. Under the code for pharmaceutical company
representatives, Medicines Austr
by alia must disclose support, incentives and other benefits provided
to prescribing doctors by pharmaceutical companies.
This Code of Conduct has been endorsed by the ACCC, who can also enforce the code if required.
Page 6 of 8
FOI 5328 IR - Document 8
The highly competitive business means sales representatives typical y work off commissions with
incentives to increase the volume of products used and the use of more expensive devices. Research
suggests company representatives also spend time in clinical areas, attend surgical procedures, and
offer technical support 24 hours a day. Patient consent may not be given for sales representatives to
be in clinical settings and company representatives may be involved in hospital purchasing processes
as a source of product information, free samples, as well as driving in-house evaluation and training
on the product.
This results in a dual role for device company representatives with potential y conflicting interests:
working as a commissioned sales representative while also providing advice on medical treatment.
We define a sales representative as any person present in a clinical area whose salary is paid by a
medical device company, regardless of what their title is or what they claim to be doing. As a team
of Australian academics argued in 2018, ‘This duality raises the concern that clinical decision-making
may be unduly influenced by commercial imperatives’, and it creates ethical concerns about the
impacts on healthcare costs, the outsourcing of expertise, and issues of accountability and informed
consent.
A class action brought against Johnson & Johnson in Australia over its vaginal mesh implants
demonstrated how some of these activities can jeopardise clinical care. Internal documents dating
from 2009 show Johnson & Johnson representatives used Lamborghinis a
under nd ski trips
Care among other
incentives to influence doctors as they rushed a class of implants to market and encouraged
inexperienced surgeons trained by company representatives to use them
1982 . This outsourcing of clinical
Aged
expertise meant some women later found it hard to find surgeons qualified to remove the defective
released
Act
implants that caused widespread pain and suffering in Australia. and
The potential impact on healthcare costs has been do
been cumented. In 2013, a 1-year retrospective
review of medical records of patients who had percutaneous coronary intervention at a Canadian
has
Health
teaching hospital showed the presence of device representatives was associated with significantly
Information
of
higher costs of balloons and stents per case, driven by the higher costs of the stents selected.
of
We also need improved controls on medical device company representatives entering clinical areas,
including informed patient consent for their presence, and ful disclosure of any benefits they
document
provide to doctors or hospitals. An enforceable Code would help ensure decisions about the use of
Department
medical devices are ful y tra
This nsparent, and
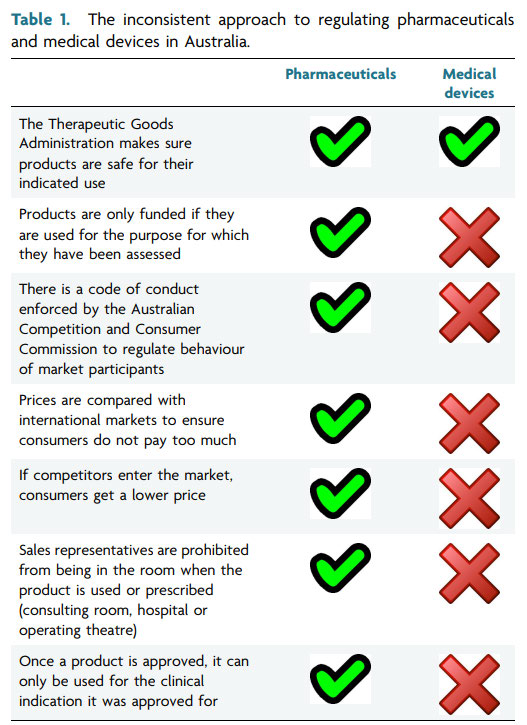
Freedom solely based on clinical considerations. The table below
outlines differences between the cur
the rent oversight of pharmaceutical and medical device industries
the
in Australia.
by
Page 7 of 8
FOI 5328 IR - Document 8
under Care
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the the
by
Page 8 of 8














