FOI 5155 - Document 1
s47G
response to MSAC CA 1530
Purified human alpha1-proteinase inhibitor
1. “Minimum clinically important differences for the primary outcome in the core randomised
controlled trials (RCTs), i.e. Computed tomography (CT)-measured lung density, are not established
in the literature…” [MSAC CA 1530, p1]
Lung CT densitometry changes have proven to be the most sensitive marker of disease progression in
patients with A1PI deficiency and COPD as compared to pulmonary function tests or quality of life
assessments (Dirksen 2009, Chapman 2015). However, in absence of an established minimum
clinically important difference (MCID) for lung density decline rates, the results seen in the RAPID and
EXACTLE trials may be difficult to interpret. To help address this issue, a group of renowned A1PI
researchers in Birmingham, UK are currently working to establish the MCID based on the CT density
outcomes from the placebo-controlled trials (Dirksen 1999, Dirksen 2009, Chapman 2015). The
researchers recently proposed an MCID of -2.89 g/L (95% CI: -2.59, -3.25) at the American Thoracic
Society conference held in May 2018 (Crossley et al 2018).
the
Based on the annual preservation of lung tissue (0.74 g/L/year) demonstrated in the RAPID trial in
favor of A1PI therapy, the proposed MCID would be achieved within 3.9 years as compared to an
under
(CTH) Care.
untreated patient. As the treatment effect was robust and largely consistent between the RAPID and
RAPID OLE trials in the Early Start patients who received 4-years of weekly infusions, a patient
continuously treated with A1PI 60 mg/kg each week can reasonably expect to maintain a reduced rate
1982 Aged
of lung density decline well beyond the point at which the proposed MCID has been reached,
released
demonstrating a worthwhile clinical improvement in this rare and often fatal disease.
Act and
2. “No significant differences were observed between A1PI and placebo for the remaining
been
effectiveness outcomes.” [MSAC CA 1530, p1]
has
Health
Demonstrating clinical efficacy in A1PI deficiency leading to COPD is challenging. It requires
of
quantitative documentation of lung function changes in a chronic and slowly progressive process that
Information
may take decades to manifest clinically (Wewers and Crystal 2013). Despite showing a significant
of
effect on lung density, the RAPID study did not show any statistical signal of efficacy in the secondary
endpoints.
document
There are several possible reasons for this: First, and importantly, the study was powered to detect
Department
the treatment effect on lung density measures, not changes in pulmonary function tests, diffusion
This Freedom
capacity of carbon monoxide (DLco), Incremental Shuttle Walking Test (ISWT), or St. George’s
the
Respiratory Questionnaire (SGRQ) scores. The sample size and trial duration reflect those necessary
to demonstrate an effect to slow the annual lung density rates, whereas it has been shown that
by
significantly more patients followed for periods longer than 2 years would be required to investigate
benefits of A1PI therapy in the secondary endpoints. Furthermore, those estimates are based on the
use of placebo which would be considered unethical for the treatment of A1PI deficiency. Secondly,
the sensitivity of the clinical endpoints to detect change is much lower compared to CT lung density;
EXACTLE, the second largest study in A1PI deficiency, established CT scans and DLco as the most
sensitive measures.
s47G
COMMERCIAL-IN-CONFIDENCE
1
Page 1 of 3
FOI 5155 - Document 1
s47G
response to MSAC CA 1530
Purified human alpha1-proteinase inhibitor
3. “A1PI meets three of the four criteria warranting rule of rescue. It is unclear whether the proposed
service provides worthwhile clinical improvement.” [MSAC CA 1530, p146]
s45
The recent work by Crossley et al to describe
the MCID for CT density decline provides further clinical context for the results seen in the RAPID trial,
and further demonstrates that A1PI offers worthwhile clinical improvement when evaluated across
the appropriate time horizon, noting that A1PI deficiency is a chronic and slowly progressive disease.
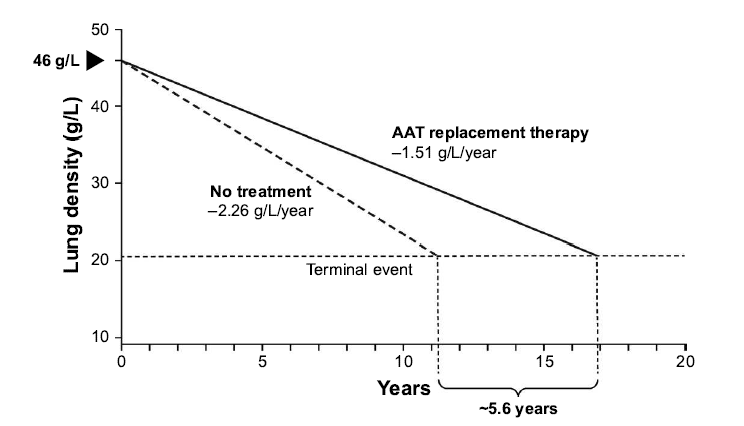
Furthermore, evidence from a post hoc analysis of the RAPID programme suggests a mortality benefit
following A1PI therapy. During the RAPID programme, the time required for progressive emphysema
to develop into respiratory crisis was used to simulate the life-years gained as a result of A1PI therapy.
the
Respiratory crisis was defined as death, lung transplant or a crippling respiratory condition. Seven
patients withdrew with an average terminal lung density of 20 g/L. Using the average baseline lung
density for all patients (46 g/L) and the rate of decline in lung density in A1PI versus placebo-treated
under Care.
patients, the projected time to terminal lung density was 16.9 years for those receiving A1PI therapy,
(CTH)
compared with 11.3 years in the placebo group (Figure 1). This indicates a gain in life-years of 5.6 years
with A1PI therapy (McElvaney et al 2017). Although conducted in a small sample size, these data are
1982 Aged
supported by results from the National Heart, Lung, and Blood Institute observational study showing
released
that patients receiving A1PI therapy had a greater survival than those not receiving treatment (Alpha-
Act and
1-Antitrypsin Deficiency Registry Study Group, 1998).
been
Figure 1
Extrapolation of the effect of A1PI replacement therapy on the predicted time to
reach terminal respiratory function in RAPID-RCT.
has
Health
Information
of
of
document
This Freedom
Department
the
by
Source: Chapman et al 2018
International Journal of COPD 18(13): 419-432
No comments on the economic evaluation or financial implications are provided in this response as
Section C, D, E were redacted from the report provided to s47G
due to the commercial in
confidence nature of the material.
s47G
COMMERCIAL-IN-CONFIDENCE
2
Page 2 of 3
FOI 5155 - Document 1
s47G
response to MSAC CA 1530
Purified human alpha1-proteinase inhibitor
REFERENCES
Alpha-1-Antitrypsin Deficiency Registry Study Group (1998). Survival and FEV1 decline in individuals
with severe deficiency of alpha1-antitrypsin. The Alpha-1-Antitrypsin Deficiency Registry
Study Group,
Am J Respir Crit Care Med, 158(1), pp. 49-59.
Chapman, K., Burdon, J., Piitulainen, E., Sandhaus, R., Seersholm, N., Stocks, J., Stoel, B., Huang, L.,
Yao, Z., Edelman, J. & McElvaney, N. (2015). Intravenous augmentation treatment and lung
density in severe Alpha-1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-
controlled trial,
Lancet 386(9991), pp. 360-368
Chapman, K. R., Chorostowska-Wynimko, J., Rembert Koczulla, A., Ferrarotti, I., & McElvaney, N. G.
(2018). Alpha 1 antitrypsin to treat lung disease in alpha 1 antitrypsin deficiency: recent
the
developments and clinical implications.
Int J Chron Obstruct Pulmon Dis 13: 419–432.
Crossley, D., Subramanian, D., Stockley, R. A., & Turner, A., M. (2018). Proposal and validation of a
under
minimal clinically important difference (MCID) for annual pulmonary CT density decline.
(CTH) Care.
American Journal of Respiratory and Critical Care Medicine 197:A3905
Dirksen, A., Dijkman, J. H., Madsen, F., Stoel, B., Hutchison, D. C., Ulrik, C. S., Skovgaard, L.T., Kok-
1982 Aged
Jensen, A.,Rudolphus, A., Seersholm, N., Vrooman, H. A., Reiber, J. H., Hansen, N.C.,
released
Heckscher, T., Viskum, K. & Stolk, J. (1999). A randomized clinical trial of alpha(1)-antitrypsin
Act and
augmentation therapy,
Am J Respir Crit Care Med 160 (5 Pt 1), pp. 1468-1472.
been
Dirksen, A., Piitulainen, E., Parr, D.G., Deng, C., Wencker, M., Shaker, S.B. & Stockley, R.A. (2009).
Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-
has
Health
antitrypsin deficiency.
Eur Respir J 33(6), pp. 1345-1353.
Information
of
McElvaney, N.G., Burdon, J., Holmes, M., Glanville, A., Wark, P. A., Thompson, P. J., Hernandez, P.,
of
Chlumsky, J., Teschler, H., Ficker, J. H., Seersholm, N., Altraja, A., Makitaro, R., Chorostowska-
Wynimko, J., Sanak, M., Stoicescu, P. I., Piitulainen, E., Vit, O., Wencker, M., Tortorici, M. A.,
Fries, M., Edelman, J. M & Ch
document apman, K. R. (2017). Long-term efficacy and safety of alpha1
proteinase inhibitor treatment for emphysema caused by severe alpha1 antitrypsin
Department
deficiency: an open-label extension trial (RAPID-OLE), Lancet Respir Med, 5(1), pp. 51-60.
This Freedom
the
Wewers, M. D., & Crystal, R. G. (2013). Alpha-1 Antitrypsin Augmentation Therapy. COPD: Journal of
Chronic Obstructive Pulmonary Disease 10(S1): 64-67
by
s47G
COMMERCIAL-IN-CONFIDENCE
3
Page 3 of 3
FOI 5155 - Document 1.1
12
45678
9
4
5
7
656
595
!"
#
$""
!%
&!%'()
&(
*+
,-
+.
/&0
1-
+.
&
/#
2
3
45
/0
6*)
7#
!8%'
295
:
0
$%
;
<!
=!%9%
!%
=%
#
6>?@?ABC
BDE
FBC
G
EBHG
?D
?I
B
G
DG
JBC
4C
G
DG
KBC
C
L
J@?>H
BDH
7G
I
IM>MDKM
N4
7O
I
?>
DDPBC
6PC
J?DB>L
4
7MDAG
HL
7MKC
G
DM
;Q
=#
!""5
(+)
;Q
$8R#
%
%*)
SQ
/Q
$
!T5
(U)
/Q
&Q
8#
%#
1V
+/;/
!W
)
X%
9#
"
(
4!"Y
5
"
6
#
%<)
6
#
%<)
X%
'
Z
%<'!)
*S!(5
;#
R(
4!"Y
5
)
;#
R()
X%
'
Z
%<'!)
U28%<
[
%9"
<
!%
X%
)
\8%
]5
^R
4!"Y)
6
#
%<)
X%
'
Z
%<'!)
1/;/
!W
)
\8%
]5
^R
4!"Y
5
)
6
#
%<)
X%
'
Z
%<'!Qthe
4?>>MA@?DEG
D_
BPH
`?>a
A
MJBG
C
8
EG
BDBb
K>?AAC
MLcdD`Ab
DMH
S
!%5
-
=!Y8
'
!!<#
Y(
3
=:
'%"
!
#
(
"
R%
8"'
"
Y#
#
(
!8
!
under
"8#
%
"9#
5
S%'!
"'
Y5
R!
=!%
#
!5'
#
5
"
3
S=":
!W
/5
Y
!%
/
(CTH) 8<%
Care. !%
#
Y(
3
//:
!
""""
5
!#
!%
!W
5
8%<
'%"
(
'5
%Q
/%
&=[
;
W
!#
=
'%"
(
e!85
'
Y#
!9
'
!#
5
#
(
!W
'%"
(
%<"
%(
Y#
9
"
9
%<
8%#
%
5
%
5
Y
Q
#
#
e!
#
!<%
"'
!'"
W
!#
Y#
!Y!"
%<
%
&=[
;V
%5
(
1982 %!#%
Aged ''"#R8!%
!'Q
f
!
'
#
%
%'
95
'
%
&=[
;
W
!#
=
'%"
(
released '5%%Y%"e//;
8"
%<
R!
#
!<%
"'
!'"Q
"
e!85
'
5
#
W
(
W
!W
Act //)%
and '!#!'%W(!"
Y
%
"
e
"
<%
W
%
'5
%
e!
(
%'
%
#
9%
!%Q
&
!'"-
g!#
'
"
#
R8
!%
!')
been
"
8'
"
e#
"!8<
#
Y!#
'
%
%'
"
%'#
'
'9
!%
!W
=
'%"
(
3
"
"8#
'
R(
+.
#
%
5
!
%
V
;+.
<
2:
R"5
%
%'
e
%%85
%<Q
"
e#
%
8"'
!
has
Health
5
85
&=[
;
8"
%<
U
9#
!%"
!W
'
"
#
R8
!%
!'-
"
%'#
'
#
#
!#
!W
of
"8#
%
3
$]&:
)
#
5
R5
%<
%'h
3
S=[
:
)
%'
W
"
^
3
]$:
Q
g!#
%!#
Information
!')
%(
YY#
"
#
Y!#
'
%%85
=
'%"
(
%<
e
#
5
9
%<
%
g]i+
"
of
"8#
'
%
5
"
e
!8
hY!"8#
!
%
%
#
9%
!%
3
Y5
R!
#
":
e#
#
9
e'Q
&=[
;
e"
%
95
'
'
8"
%<
6
#
%<
//
!!#
Q
%
"
e!
'
#
9'
e!
!#
!#
=
"%"
Y5
8"
5
"
#
g]
document i+"8#%"!9#5"#(#"e#'%W'Q
/%%85
"5
!Y
g]i+
3
5
":
e"
5
85
'
%'
!Y#
'
e
#
"Y
9
%%85
=
'%"
(
Department
%<
%'
!Y#
This 'R(5%##<#""!%%5(""QS"85"-g<8#!%58"#"&=[;
Freedom
%'
#
!%W
'%
%
#
95
"Q
!%W
'%
%
#
95
"
W
#
!
%!#
!'
%!Y""'
the
!"
!W
$]&
%'
S=[
Q
7
9%
Y#
!Y!"'
&=[
;
"!85
'
!#
<
%
W
#
!
9#
(
!W
!'")
"
#
"!%R5
by !Y#!Y!"&=[;W!#='%"("0*Qj,<2"""''5
"
W
#
!
#
'
"
#
R8
!%
!'")
%'
"
5
e
%
!%W
'%
%
#
95
"
!W
%!#
!'Q
=!%5
8"
!%-
Y#
!Y!"'
&=[
;
W
!#
=
'%"
(
%
Y
%
"
e
//;
"
0
*Q
j,<
2Q
i5
8"
%
h""
!W
"
%
Y
%
"
8%'#
"8#
9
5%
e!85
'
%'
#
Y
'
'%"
(
'5
%)
e
(
R
!%
%'
!%
W
!#
//
8<%
!%
#
Y(Q
Page 1 of 2
FOI 5155 - Document 1.1
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
12
3
5637
8
597
2
3
6
2
3
2
7
157
2
93
Information
of
! "#$%&'()
'*+,
-*&.
/
0!
0!"$$,1112
"!
3
450"6
2
47
806
09!
":!
/
5
of
document
This Freedom
Department
the
by
Page 2 of 2
FOI 5155 - Document 2
14 September 2018
s47G
response to MSAC Contracted Assessment 1530
Overall, the need for treatment of patients with alpha-1 antitrypsin deficiency (AATD), as well as the
clinical evidence for augmentation therapy with alpha-1 proteinase inhibitor (A1PI), is well summarised
in the Assessment Report.
With respect to the findings, s47G notes that the Assessment Report concluded that for the outcome
of computed tomography (CT)-lung density, a statistically significant treatment effect was observed.
Given that the aim of treatment with A1PI is to slow the rate of decline in lung function, improve quality
of life and extend the patient’s life-expectancy, this is a critically important finding. Indeed, the
Assessment Report concurred with s47G submission that there is evidence supporting a correlation
between CT-lung density decline, mortality and functional outcomes. While s47G acknowledges the
uncertainty around the magnitude of the benefit, large randomised clinical trials are required to provide
more accurate estimates. Due to the rarity of AATD, this is no longer ethically possible with the
availability of A1PI therapies that are bioequivalent (i.e. Prolastin-C and Zemaira). Nonetheless, based
on the totality of the clinical evidence, it is reasonable to expect that treatment with A
the 1PI will slow rate
of decline in lung function, improve quality of life and survival in Australian clinical practice.
With interest, s47G notes the Consumer Impact Statement from the foundation for patients with
under
AATD which highlights its intention to establish an Australian register for patients to better understand
(CTH) Care.
the epidemiology of the disease if the A1PI therapies are funded through the NBA. In addition to the
other consumer impact statements regarding the need for therapies with a demonstrated clinical
benefit in AATD, s47G welcomes the thorough consideration of the Rule of Rescue in the Assessment
1982 Aged
Report including the acknowledgements that there is currently no treatment for patients with AATD, as
well as the small number of patients that suffer from this severely de
released bilitating disease that is associated
with significant reduction in survival.
Act and
s47G
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Page 1 of 1

FOI 5155 - Document 3
s47F
s47F
s47F
s47F
s47F
s47G
the
under
(CTH) Care.
Aged
released 1982
Act and
been
s47G
s47G
has
Health
Information
of
s47G
of
s47G document
This Freedom
Department
the
s47F
by
s47F
s47F
s47F
s47G
Page 7 of 12

FOI 5155 - Document 3
s47F
s47F
s47F
s47G
s45
s45
s47F
the
s47G
under
s47G(CTH) Care.
Aged
released 1982
s47F
Act and
been
s47G
has
Health
Information
of
of
document
s47F
This Freedom
Department
s47F
the
s47F
s47F
by
s47F
s47G
s47G
Page 9 of 12

FOI 5155 - Document 3
s47G
s47G
s47F
s47F
s47F
s47F
the
under
(CTH) Care.
Aged
released 1982
Act and
been
s47G
s47G
has
Health
Information
of
of
document
This Freedom
Department
the
by
Page 10 of 12

FOI 5155 - Document 3
s47G
s47G
s47G
the
under
s47G
(CTH) Care.
Aged
released 1982
Act and
been
s47G
has
Health
Information
of
of
s47G
s47G
document
s47G
This Freedom
Department
the
by
s47G
s47G
Page 11 of 12

FOI 5155 - Document 3
s47G
the
under
(CTH) Care.
s47G
Aged
released 1982
Act and
s47G
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
s45,
s47G
s47G
s45, s47G
s45,
s45, s47G
s47G
s45, s47G
s47G
s47G
Page 12 of 12

FOI 5155 - Document 3.1
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Page 1 of 48

FOI 5155 - Document 3.1
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Page 2 of 48
FOI 5155 - Document 3.1
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Page 3 of 48
FOI 5155 - Document 3.1
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Page 4 of 48

FOI 5155 - Document 3.1
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Page 5 of 48

FOI 5155 - Document 3.1
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Page 6 of 48

FOI 5155 - Document 3.1
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Page 7 of 48

FOI 5155 - Document 3.1
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Page 8 of 48

FOI 5155 - Document 3.1
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Page 9 of 48

FOI 5155 - Document 3.1
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Page 10 of 48

FOI 5155 - Document 3.1
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Page 11 of 48

FOI 5155 - Document 3.1
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Page 12 of 48
FOI 5155 - Document 4
Purified human
alpha1-proteinase
inhibitor for the
treatment of alpha1-
proteinase inhibit
the
or
deficiency, leading
to chronic
under
(CTH) Care.
obstructive
pulmonary disease
1982 Aged
released
Act and
been
has
Health
Au
of
gust 2018
Information
of
document
MSAC application no. 1530
This Freedom
Department
the
Assessment report
by
Page 1 of 218

FOI 5155 - Document 4
© National Blood Authority 2018
Internet site http://www.msac.gov.au/
This work is copyright. You may download, display, print and reproduce this material in unaltered form only
(retaining this notice) for your personal, non-commercial use or use within your organisation. Apart from any
use as permitted under the Copyright Act 1968, al other rights are reserved. Requests and inquiries
concerning reproduction and rights should be addressed to Commonwealth Copyright Administration,
Attorney-General's Department, Robert Garran Offices, National Circuit, Barton ACT 2600 or posted at
http://www.ag.gov.au/.
Electronic copies of the report can be obtained from the Medical Service Advisory Committee’s Internet site at
http://www.msac.gov.au/
the
Enquiries about the content of the report should be emailed to xxx@xxxxxx.xxx.xx.
The technical information in this document is used by the Medical Services Advisory Committee (MSAC) to
inform its deliberations. MSAC is an independent committee that has been established to provide advice to
under
the Minister for Health on the strength of evidence available on new and existing medical technologies and
(CTH) Care.
procedures in terms of their safety, effectiveness and cost effectiveness. This advice wil help to inform
government decisions about which medical services should attract funding under Medicare.
1982 Aged
MSAC’s advice does not necessarily reflect the views of al individuals who participated in the MSAC
released
evaluation.
Act and
This report was prepared by Research and Evaluation, i
been ncorporating ASERNIP-S of the Royal Australasian
Col ege of Surgeons, and eSys Development Pty Limited. The report was commissioned by the Australian
Government Department of Health.
has
Health
of
The suggested citation for this document is:
Information
of
Vreugdenburg TD, Scarfe AJ, Ma N, Jacobsen JH, McLeod R, Tivey D (2018). Purified human alpha1-proteinase
inhibitor for the treatment of alpha1-proteinase inhibitor deficiency, leading to chronic obstructive pulmonary
disease. MSAC Application 1530, Assessm
document ent Report. Commonwealth of Australia, Canberra, ACT.
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
ii
Page 2 of 218
FOI 5155 - Document 4
CONTENTS
Contents
............................................................................................................................. iii
Tables ....................................................................................................................................... v
Boxes ....................................................................................................................................... x
Figures .................................................................................................................................... xi
List of terms ........................................................................................................................... xiii
Executive Summary ...................................................................................................................... 1
Alignment with agreed PICO Confirmation .............................................................................
the
2
Proposed medical service ........................................................................................................ 2
Proposal for public funding ..................................................................................................... 2
under Care.
Population ...............................................................................................................................
(CTH)
2
Comparator details .................................................................................................................. 2
Clinical management algorithm(s) ..........................................................................................
1982 Aged
3
released
Clinical claim ............................................................................................................................ 3
Act and
Approach taken to the evidence assessment .......................................................................... 3
been
Characteristics of the evidence base ....................................................................................... 3
Results ..................................................................................................................................... 3
has
Health
Translation issues .................................................................................................................... 6
Information
of
Economic evaluation ............................................................................................................... 6
of
Estimated extent of use and financial implications ................................................................. 8
Consumer impact summary .................................................................................................... 9
document
Other relevant considerations ................................................................................................. 9
Department
Section A
Cont
This
ext ................................................................................................................ 10
Freedom
A.1. Items in the ag
the reed PICO Confirmation .................................................................... 10
A.2. Propose
by d medical service ......................................................................................... 10
A.3. Proposal for public funding ....................................................................................... 13
A.4. Proposed population ................................................................................................. 13
A.5. Comparator details ................................................................................................... 16
A.6. Clinical management algorithm ................................................................................ 17
A.7. Key differences in the delivery of the proposed medical service and the
main comparator ................................................................................................................... 20
A.8. Clinical claim.............................................................................................................. 20
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
iii
Page 3 of 218
FOI 5155 - Document 4
A.9. Summary of the PICO ................................................................................................ 20
A.10. Consumer impact statement .................................................................................... 21
Section B
Clinical evaluation ................................................................................................ 23
B.1. Literature sources and search strategies .................................................................... 23
B.2. Results of literature search ......................................................................................... 23
B.3. Risk of bias assessment ............................................................................................... 29
B.4. Characteristics of the evidence base........................................................................... 36
B.5. Outcome measures and analysis ................................................................................. 38
Primary effectiveness outcomes ........................................................................................... 38
the
Secondary effectiveness outcomes ....................................................................................... 44
B.6. Results of the systematic literature review ................................................................ 48
Is it safe? ................................................................................................................................ 48
under
(CTH) Care.
Is it effective (RCT evidence)? ............................................................................................... 60
B.7. Extended assessment of harms ................................................................................... 70
1982 Aged
B.8. Interpretation of the clinical evidence ........................................................................ 71
released
Section C
Translation Issues ................................................................................................
Act
. 74
and
C.1. Overview ..................................................................................................................... 74
been
C.2. Applicability translation issues .................................................................................... 74
Health
C.3. Selection of utility value issu
has es ................................................................................... 79
of
C.4. Extrapolation translation issues .................................................................................. 90
Information
C.5 Relationship of each pre-modelling study to the economic evaluation .................... 96
of
Section D
Economic Evaluation ............................................................................................. 98
document
D.1. Overview ................................................................................................................... 98
D.2. Populations and settings ........................................................................................... 99
This Freedom
Department
D.3. Structure and rationale of the economic evaluation .............................................. 101
the
D.4. Inputs to the economic evaluation ......................................................................... 120
by
D.5. Results of the Economic Evaluation ........................................................................ 126
D.6. Sensitivity analyses ................................................................................................. 129
Section E
Financial Implications ......................................................................................... 135
E.1. Justification of the selection of sources of data........................................................ 135
E.1. Costs to the NBA of the proposed therapy over five years....................................... 141
E.2. Changes in use and cost of other medical services ................................................... 141
E.3. Overall financial implications .................................................................................... 142
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
iv
Page 4 of 218
FOI 5155 - Document 4
E.4. Identification, estimation and reduction of uncertainty ........................................... 142
Section F
Other relevant considerations ............................................................................. 144
Access considerations .......................................................................................................... 144
Dosing considerations ......................................................................................................... 145
Ethical considerations: Rule of rescue ................................................................................. 146
Appendix A Clinical Experts and Assessment Group ............................................................... 147
Clinical experts consulted during the preparation of this report ........................................ 147
Assessment group ............................................................................................................... 147
Appendix B Search strategies ................................................................................................ 148
the
Appendix C Studies included in the Systematic Review .......................................................... 153
Appendix D Evidence Profile Tables ....................................................................................... 170
under
Appendix E
Care.
Excluded Studies ................................................................................................
(CTH)
. 190
References .......................................................................................................................... 193
Aged
released 1982
T
Act
ABLES
and
been
Table 1
Balance of clinical benefits and harms of A1PI relative to placebo as
measured by the critical patient-relevant outcomes in the key studies ................ 5
has
Health
Table 2
Summary of the economic evaluatio
of n ................................................................... 6
Information
Table 3
Incremental Cost Effectiveness Ratio (1,000-patient cohort) ................................ 7
of
Table 4
Drivers of the economic model .............................................................................. 8
document
Table 5
Total costs to the NBA associated with AT ............................................................. 9
Table 6
Approved augmentation therap
Department ies and their indications .................................... 12
This Freedom
Table 7
Studies evaluating the biocompatability of A1PI therapies ................................. 12
the
Table 8
Serum A1PI levels associated with normal and SZ or ZZ allele variations
by
known to increase the risk of emphysema (Hatipoglu and Stoller 2016) ............ 15
Table 9
Trials (and associated data) presented in the assessment report ....................... 25
Table 10 Details of clinical trials identified on Clinicaltrials.gov ......................................... 27
Table 11 Details of clinical trials identified on EU Clinical Trials Registry ........................... 28
Table 12 Details of clinical trials identified on WHO International Clinical Trials
Registry Platform .................................................................................................. 28
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
v
Page 5 of 218
FOI 5155 - Document 4
Table 13 Patient flow in randomised controlled trials ........................................................ 32
Table 14 Key features of the included RCTs comparing A1PI augmentation
therapy with placebo ........................................................................................... 36
Table 15 Key features of the included studies assessing alpha-1 antitrypsin
augmentation for safety outcomes ...................................................................... 37
Table 16 Primary outcomes and statistical analyses of the randomised and non-
randomised controlled trials ................................................................................ 39
Table 17 Studies assessing correlation between CT lung density and function
markers in AATD patients ..................................................................................... 41
the
Table 18 Secondary outcomes and statistical analyses of the direct randomised
trials ...................................................................................................................... 44
Table 19 Results of death due to adverse events across the included randomised
under Care.
controlled trials and single-arm studies ...............................................................
(CTH)
49
Table 20 Results of severe adverse events across the included randomised
controlled trials and single-arm studies ...............................................................
1982 Aged
50
released
Table 21 Results of treatment-related adverse events across the included
Act and
randomised controlled trials and single-arm studies ........................................... 52
been
Table 22 Results of dyspnoea across the randomised controlled trials and single-
arm studies ........................................................................................................... 53
has
Health
Table 23 Results of discontinuation due to a
of dverse events across the included
Information
randomised controlled trials and single-arm studies ........................................... 55
of
Table 24 Hospitalisation due to adverse events across the included studies .................... 56
Table 25 Results of any ad
document verse events across the included randomised controlled
trials and single-arm studies ................................................................................ 57
This
Department
Table 26 Results of sever
Freedom e adverse events across the RCTs and non-controlled
trials treatin
the g with Zemaira and PROLASTIN-C .................................................... 59
by
Table 27 Results of mortality across the randomised controlled trials at 24
months ................................................................................................................. 60
Table 28 Results of mortality across the non-randomised controlled trials ....................... 61
Table 29 Results of exacerbations across the direct randomised controlled trials ............ 62
Table 30 Results of exacerbations across the non-randomised trials ............................... 62
Table 31 Results of hospitalisations across the direct randomised controlled trials ......... 63
Table 32 Results of quality of life across the direct randomised controlled trials† ............ 63
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
vi
Page 6 of 218
FOI 5155 - Document 4
Table 33 Results of quality of life across the non-randomised controlled trials† ............... 64
Table 34 Results of shuttle walk distance (metres) in the direct randomised
controlled trial ...................................................................................................... 65
Table 35 Results of change in FEV1 (% predicted or mL) across the direct
randomised controlled trials† ............................................................................... 66
Table 36 Results of change in FEV1 (% predicted or mL) across the non-
randomised controlled trials ................................................................................ 67
Table 37 Results of CT-measured lung density (total lung capacity, g/L per year)
across the direct randomised controlled trials .................................................... 68
the
Table 38 Results of carbon monoxide diffusing capacity across the direct
randomised controlled trials ................................................................................ 69
Table 39 Results of carbon monoxide diffusing capacity across the non-
under Care.
randomised controlled trials ................................................................................
(CTH)
69
Table 40 Results of lung infections in non-randomised control ed trials ........................... 70
1982 Aged
Table 41 Results of hospitalisation days in non-randomised controlled trials .................. 70
released
Table 42 Balance of clinical benefits and harms of A
Act 1PI relativ
and e to placebo as
measured by the critical patient-relevant outcomes in the key studies .............. 73
been
Table 43 Outline of Section C issues being addressed ........................................................ 74
has
Health
Table 44 Comparison of the RAPID trial’s patient population and the proposed
of
listing (Chapman et al. 2015) ................................................................................ 76
Information
Table 45 Comparison of Di
of rksen and EXACTLE patient population and the
proposed listing .................................................................................................... 77
document
Table 46 Comparison of the UK registry patient population and the proposed
listing .................................................................................................................... 78
This Freedom
Department
Table 47 Search strategy for AATD utility literature review ............................................... 79
the
Table 48 Results of AATD utility literature review .............................................................. 80
by
Table 49 Studies identified outlining utilities for AATD and COPD states .......................... 80
Table 50 EQ-5D values stratified by FEV1% predicted, obtained from the UK
ADAPT registry ...................................................................................................... 81
Table 51 Selected EQ-5D values stratified by GOLD (FEV1%) states from Moayeri
et al. 2016 ............................................................................................................. 83
Table 52 Selected utility values from COPD models outlined by Hoogendoorn et
al. 2017 ................................................................................................................. 85
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
vii
Page 7 of 218
FOI 5155 - Document 4
Table 53 Utilities for lung transplantation .......................................................................... 87
Table 54 Summary of utility inputs for the Section D cost-effectiveness mode ................ 89
Table 55 Goodness of fit and parameters for FEV 1 >50 survival models .......................... 93
Table 56 Goodness of fit and parameters for FEV 1 <50 no decline survival models......... 94
Table 57 Goodness of fit and parameters for FEV1 <50 slow decline survival
models .................................................................................................................. 95
Table 58 Goodness of fit and parameters for FEV 1 <50 rapid decline survival
models .................................................................................................................. 96
Table 59 Summary of results of pre-model ing studies and their uses in the
the
economic evaluation ............................................................................................ 96
Table 60 Comparison between eligibility criteria in the RAPID study and
circumstances of use ............................................................................................
under
99
(CTH) Care.
Table 61 Baseline disease severity – RAPID population; baseline disease severity
in the model ....................................................................................................... 100
1982 Aged
Table 62 Summary of the economic evaluation ............................................................... 101
released
Act and
Table 63 Search terms used .............................................................................................. 102
Table 64 Summary of the process used t
been o identify and select studies for the
economic evaluation .......................................................................................... 103
has
Health
Table 65 Economic models assessing A1PI deficiency treatment .................................... 103
Information
of
Table 66 Summary of COPD economic model progression and mortality
of
characteristics .................................................................................................... 109
Table 67 Summary of the process used to identify and select lung transplant
document
studies for the economic evaluation .................................................................. 110
Department
Table 68
This Economic evaluations of lung transplantation ................................................... 111
Freedom
the
Table 69 Economic model health states ........................................................................... 119
by
Table 70 Baseline patient and disease characteristics of the modelled patient
cohort ................................................................................................................. 120
Table 71 Health state transition probabilities – Years 1-4 ................................................ 121
Table 72 Health state transition probabilities – Years >4 years ....................................... 121
Table 73 Health state dispositions at month 24 and month 30 and associated
transition probabilities – patients with severe depression at baseline ............. 122
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
viii
Page 8 of 218
FOI 5155 - Document 4
Table 74 Resources associated with AT and disease management costs by COPD
severity ............................................................................................................... 124
Table 75 Utility value used in the model .......................................................................... 126
Table 76 Health care costs by resource type for base-case analysis (1,000-person
cohort) ................................................................................................................ 126
Table 77 Average patient health outcomes by health state and by outcome
measure for trial analysis ................................................................................... 127
Table 78 Health outcomes by health state and by outcome measure for lifetime
analysis (Per patient) .......................................................................................... 128
the
Table 79 Incremental Cost Effectiveness Ratio (1,000-patient cohort) ............................ 128
Table 80 Sensitivity analysis for lifetime analysis ............................................................. 132
Table 81 Key drivers of the economic model ....................................................................
under
134
(CTH) Care.
Table 82 Summary of the key assumptions used in the financial impact
assessment ......................................................................................................... 136
1982 Aged
Table 83 Population eligible for augmentation therapy with A1PI in Australia ............... 138
released
Act and
Table 84 Uptake of augmentation therapy with A1PI in Australia ................................... 140
Table 85 Estimated AT vial usage in Australia,
been 2019-2023 ............................................... 141
Table 86 Estimated financial impact to the National Blood Authority; total
has
Health
augmentation therapy market ........................................................................... 141
Information
of
Table 87 Estimated financial impact to MBS from augmentation therapy listing ............ 142
of
Table 88 Estimated financial impact to government from augmentation therapy
listing .................................................................................................................. 142
document
Table 89 Net government cost sensitivity analysis ........................................................... 143
This
Department
Table 90 Studies evaluat
Freedom ing different doses of A1PI therapies ........................................ 145
the
Table 91 PubMed Search Strategy .................................................................................... 149
by
Table 92 Embase Search Strategy ..................................................................................... 150
Table 93 Cochrane Search Strategy .................................................................................. 150
Table 94 Clinicaltrials.gov Search Strategy ....................................................................... 150
Table 95 Cochrane Central Register of Controlled Trials Search Strategy ........................ 151
Table 96 EU Clinical Trials Registry Search Strategy ......................................................... 151
Table 97 WHO International Clinical Trials Registry Platform Search Strategy ................ 151
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
ix
Page 9 of 218
FOI 5155 - Document 4
Table 98 Current Controlled Trials MetaRegister Search Strategy ................................... 151
Table 99 Australian New Zealand Clinical Trials Registry Search Strategy ....................... 152
Table 100 CEA Registry Search Strategy ............................................................................. 152
Table 101 Characteristics of randomised controlled trials included in the systematic
review to assess efficacy .................................................................................... 153
Table 102 Characteristics of RCT studies used in the systematic literature review to
assess safety ....................................................................................................... 156
Table 103 Characteristics of non-randomised controlled trials used in the systematic
literature review to assess efficacy .................................................................... 159
the
Table 104 Characteristics of single arm studies used in the systematic literature
review to assess safety ....................................................................................... 165
Table 105 Evidence profile table of effectivness outcomes for A1PI c
under ompared to
(CTH) Care.
placebo for patients with severe AATD and emphysema .................................. 170
Table 106 Evidence profile table of safety outcomes for A1PI compared to placebo
1982 Aged
for patients with severe AATD and emphysema ................................................ 174
released
Table 107 Modified quality appraisal of included c
Act ase serie
and s investigations
according to the IHE Quality Appraisal of Case Series Studies (Guo et al.
been
2016)................................................................................................................... 177
Health
Table 108 Risk of bias in non–ran
has domised studies comparing A1PI augmentation
therapy and best supportive care or
of placebo .................................................... 178
Information
Table 109 Safety outcomes reported in RCT studies .......................................................... 179
of
Table 110 Safety outcomes reported in single arm studies ............................................... 183
document
Department
BOXES This Freedom
the
Box 1
Criteria for identifying and selecting studies to determine the safety of
by
purified human A1PI for the treatment of A1PI deficiency, leading to
COPD ..................................................................................................................... 20
Box 2
Criteria for identifying and selecting studies to determine the
effectiveness of purified human A1PI for the treatment of A1PI
deficiency, leading to COPD ................................................................................. 21
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
x
Page 10 of 218
FOI 5155 - Document 4
FIGURES
Figure 1
Simplified schematic of the pathway to lung and liver disease associated
with A1P1 deficiency (Fregonese and Stolk 2008) ............................................... 14
Figure 2
Current clinical management algorithm for patients with emphysema
and FEV1 <80% ..................................................................................................... 18
Figure 3
Proposed clinical management algorithm for patients with emphysema
and FEV1 <80% ..................................................................................................... 19
Figure 4
Summary of the process used to identify and select studies for the
assessment ........................................................................................................... 24
the
Figure 5
Summary of the overall risk of bias across the included studies ......................... 29
Figure 6
Risk of bias in the included randomised controlled trials .................................... 30
under Care.
Figure 7
Summary of risk of bias across the included non-randomised studie
(CTH) s ............... 34
Figure 8
Summary of risk of bias across the included single-arm studies ......................... 36
1982 Aged
Figure 9
Forest plot indicating the pooled rate of severe adverse events for A1PI
released
compared to placebo ........................................................................................... 51
Act and
Figure 10 Forest plot indicating rate of death due to adverse events in A1PI
been
patients compared to placebo ............................................................................. 53
Figure 11 Forest plot indicating discontinuation due to
Health adverse events for A1PI
has
compared to placebo ...........................................................................................
of
55
Information
Figure 12 Forest plot indicating mean changes in St George’s Respiratory
of
Questionnaire results for A1PI compared to placebo .......................................... 64
Figure 13 Forest plot indic
document ating standardised mean differnces in FEV1 for A1PI
compared to placebo ........................................................................................... 66
This
Department
Figure 14 Forest plot ind
Freedom icating changes in CT-measured lung density (g/mL) in
A1PI comp
the ared to placebo measured at 24 to 30 months follow-up.
(Chapman 2015 and Dirksen 1999 reported an annualised rate, whereas
by
Dirksen 2009 reported the change from baseline at 24 months.) ....................... 68
Figure 15 Forest plot indicating the standardised mean difference in carbon
monoxide diffusing capacity (DLCO) for A1PI compared to placebo ..................... 69
Figure 16 FEV1>50 survival models ...................................................................................... 92
Figure 17 FEV1 <50 no decline survival models ................................................................... 93
Figure 18 FEV1 <50 slow decline survival models ................................................................. 94
Figure 19 FEV1<50 rapid decline survival models ................................................................. 95
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
xi
Page 11 of 218
FOI 5155 - Document 4
Figure 20 Decision tree for Augmentation Therapy ........................................................... 115
Figure 21 AT patient distribution between health states – based on RAPID data
and parametric modelling .................................................................................. 123
Figure 22 BSC patient distribution between health states – based on RAPID data
and parametric modelling .................................................................................. 123
Figure 23 Difference between AT and BSC patient distributions across health
states by year ..................................................................................................... 124
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
xii
Page 12 of 218
FOI 5155 - Document 4
LIST OF TERMS
Acronym/Abbreviation
Definition
A1PI
Alpha-1 proteinase inhibitor
AATD
Alpha-1 anti-trypsin deficiency
AT
Augmentation therapy
ARTG
Australian Register of Therapeutic Goods the
BOS
Bronchiolitis obliterans syndrome
BODE
BMI, obstruction, dyspnoea, exercise cap
under acity
(CTH) Care.
BSC
Best supportive care
1982 Aged
COPD
Chronic obstructive pulmonary disease
released
Act
CT
Computed tomography
and
been
DLCO
Diffusing capacity for carbon monoxide
Health
EQ-5D
Euro
has qol group 5 domain questionnaire
Information
of
FEV1
Forced expiratory volume in 1 second
of
FRC
Functional residual capacity
document
FVC
Forced vital capacity
This
Department
GOLD
Freedom Global initiative for chronic obstructive lung disease
the
ICER
Incremental cost-effectiveness ratio
by
IgA
Immunoglobulin A
IPD
Individual patient data
KCO
Diffusing coefficient for carbon monoxide
MBS
Medicare benefits schedule
MCIDs
Minimal clinically important differences
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
xiii
Page 13 of 218
FOI 5155 - Document 4
MITT
Modified intention-to-treat
MSAC
Medical Services Advisory Committee
NBA
National Blood Authority
NPL
National Product List
PASC
PICO Advisory Sub-committee
PBAC
Pharmaceutical Benefits Advisory Committee
PD15
15th Percentile lung density
the
PI
Product information
under
PICO
Population, intervention, comparator, outcomes
(CTH) Care.
QALY
Quality-adjusted life year 1982 Aged
QoL
Quality of life released
Act and
RCT
Randomised control ed trials
been
SGRQ
St Georges Respiratory Questionnaire
has
Health
SMD
Standardised mean difference
Information
of
TGA
Therapeutic Goods Administration
of
TLC
Total lung capacity
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
xiv
Page 14 of 218
FOI 5155 - Document 4
EXECUTIVE SUMMARY
Main issues for MSAC consideration
• Minimum clinically important differences (MCIDs) for the primary outcome in the core
randomised controlled trials (RCTs), i.e. Computed tomography (CT)-measured lung density,
are not established in the literature. The best available evidence suggests a correlation
between CT-lung density decline and mortality and functional outcomes, however, it is
currently unclear whether, or to what extent, this translates to a clinically important impact
of augmentation therapy (AT) with Alpha-1 proteinase inhibitor (A1PI). No significant
differences were observed between A1PI and placebo for the remaining effectiveness
the
outcomes.
• Only a limited number of economic studies relating to AT cost effectiveness were identified
under
in the literature. Two American studies related resource use to expected life gain usin
Care.g USA
(CTH)
registry data. High incremental expected survival of 7+ years in non-smokers resulted in AT
appearing relatively cost effective. The RAPID trial was not powered to determine
1982 Aged
differences in forced expiratory volume in 1 second (FEV1) or mortality, so uncertainty
released
surrounds the magnitude of this clinical benefit given available trial data.
Act and
• The model ing in this assessment generated a lifetime incremental cost-effectiveness ratio
been
(ICER) of s47(1)(b) per quality-adjusted life year (QALY) and a trial period ICER of s47(1)(b) .
It is evident that most benefits accrue after the RAPID trial period. The assumption about the
has
Health
price paid for the AT product is the key driver of model results. The base cost of AT assumes
of
a price per 1,000 ml of s47(1)
s47(1)
s47(1)
Information
(b)
. This varies from (b)
to (b)
per 1,000ml vial. The estimated
ICER varies considerably from s47(1)(b) to s47(1)(b) per QALY.
of
• The estimated NBA financial cost of AT listing is presented over a six-year costing proposal
document
period and is based on a s47(1)
s47(1)
(b)
uptake rate for AT by 2023. Uptake begins at (b) and
increases by s47(1)
Department
(b)
per year. The cost to the national blood authority (NBA) for the total AT
This
market is estimated to be
Freedom s47(1)(b) in 2019, increasing to s47(1)(b) in 2023.
the
• A key uncertainty is the price of AT. Given the large contribution of the AT product itself to
by
overall resource in the economic model, variations in price have a large impact on both
financial and economic attractiveness.
This contracted assessment examines the evidence supporting the listing of purified human A1PI on
the National Product List (NPL) for blood products. The service would primarily be used in the
outpatient hospital or clinic setting for the treatment of A1PI deficiency, also known as alpha-1
antitrypsin deficiency (AATD). Some patients may be able to self-administer the intervention at
home. The target population is people with severe A1PI deficiency (defined as serum A1 ≤11 μM)
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
1
Page 15 of 218
FOI 5155 - Document 4
plus emphysema. The applicant claims that successful listing of the technology in the target
population and setting will lead to slower disease progression compared to best supportive care.
ALIGNMENT WITH AGREED PICO CONFIRMATION
This contracted assessment largely conforms to the PICO elements that were pre-specified in the
PICO Confirmation ratified by the PICO Advisory Sub-Committee (PASC). Only placebo-control ed
trials were identified for the evaluation of effectiveness outcomes (i.e. not best supportive care), and
eligible indications were broadened slightly for the evaluation of safety outcomes (i.e. not limited to
phenotype PiZZ).
PROPOSED MEDICAL SERVICE
the
The proposed medical service is for lifelong intravenous blood augmentation via weekly infusions of
purified human A1PI. The currently recommended dosing strategy is 60mg/kg p
under er week, noting that
(CTH) Care.
ongoing trials are investigating optimal dosing regimens. Augmentation therapy with A1PI is not
currently funded or reimbursed in private or public settings in Australia for this or any other clinical
indication.
Aged
released 1982
PROPOSAL FOR PUBLIC FUNDING
Act and
been
AT with A1PI is proposed for reimbursement on the National Products List (NPL), managed by the
National Blood Authority (NBA). As such, no Medicare Benefits Schedule (MBS) item descriptor is
has
Health
required.
Information
of
POPULATION
of
The intended population includes ex- or never-smoking patients with emphysema and severe A1PI
document
deficiency (serum A1 ≤ 11 μM). The frequency of Australians with PiZZ allele, which indicates the
most severely affected patients with greatly increas
Department ed risk of emphysema, is estimated at 1 in 5,584.
This Freedom
Null allele is very rare and its occurrence cannot be estimated. Based on educated estimates, the
the
number of people meeting the criteria for treatment with A1PI in Australia in 2018 was s47(1)
(b)
Considering treatment is
by lifelong and not curative, the number of patients being treated is expected
to have a moderate cumulative increase over time.
COMPARATOR DETAILS
The comparator intervention for patients with chronic obstructive pulmonary disease (COPD) is best
supportive care (BSC). Strategies for the management of stable COPD include non-pharmaceutical
strategies (pulmonary rehabilitation and physical activity), pharmacological strategies (inhaled
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
2
Page 16 of 218
FOI 5155 - Document 4
medications, corticosteroids and antibiotics), and prevention of deterioration and end-stage
strategies.
CLINICAL MANAGEMENT ALGORITHM(S)
Patients with A1PI deficiency are currently managed with BSC, which aims to provide symptomatic
relief. AT is an additive intervention to supplement BSC for patients with emphysema. The current
(Figure 2) and proposed (Figure 3) clinical management algorithms are presented in the report.
CLINICAL CLAIM
The applicant claims that, relative to best supportive care, A1PI slows disease progression in patients
the
with severe A1PI deficiency and emphysema.
APPROACH TAKEN TO THE EVIDENCE ASSESSMENT
under
(CTH) Care.
A systematic review of published and unpublished literature was undertaken. The medical literature
was searched to identify relevant studies in Embase on 23 May 2018 and in PubMed and The
1982 Aged
Cochrane Library on 24 May 2018. RCTs were appraised for risk of bias using the Cochrane RoB 2.0
released
tool, non-randomised studies were appraised using the Cochra
Act ne ROBINS-I tool, and single-arm
and
studies were appraised using the IHE checklist for observational studies.
been
CHARACTERISTICS OF THE EVIDENCE BASE has
Health
Three RCTs were identified that evaluated the effectiv
of eness of A1PI compared to placebo in 313
Information
patients. Included patients were relatively homogenous across the included studies, representing ex-
of
or never-smokers with severe A1PI deficiency (serum A1 ≤11µM) and emphysema (FEV1 25% to
80%). The included RCT outcomes were generally well conducted; however, method of allocation
document
concealment was poorly reported across all trials. Seventeen single arm studies were identified that
provided evidence on the safety of A1PI.
This Freedom
Department
RESULTS
the
by
SAFETY
Seventeen single arm studies were included for the evaluation of safety outcomes. Key safety
outcomes were: death due to adverse events, severe adverse events, and discontinuation or
hospitalisation due to adverse events.
Six deaths occurred in the eligible studies, which included 899 patients. None of these deaths were
reported to be treatment-related. Severe adverse events were also uncommon, with a median
occurrence of 2% in the patient population (range 0%-38%). Discontinuation due to adverse events
had a median occurrence of 0.5% in the patient population (range 0%-12%) across nine studies.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
3
Page 17 of 218
FOI 5155 - Document 4
Hospitalisation had a median occurrence of 1.5% in the patient population (range 0%-14%) across
four studies.
Three studies reported safety in patients treated with one of the two therapies under assessment,
Zemaira and PROLASTIN-C. Al of these studies found that rates of severe adverse events were
unchanged across intervention groups.
Fifteen studies reported any adverse event, with a rate ranging from 0% to 100% and a median of
37%. Differences between the RCTs and observational studies in the rates of any adverse event may
indicate under-reporting in the observational studies. Dyspnoea and treatment-related adverse
events were also reported. Dyspnoea occurred after AT in 12.5% of the patient population (range
0%-35%). Events reported by the authors to be treatment-related had a median occurrence of 11%
the
in the patient population (range 0%-38%).
Overall, it appears that the intervention is safe, with most events being related to the underlying
under Care.
disease.
(CTH)
EFFECTIVENESS
1982 Aged
No direct trials comparing A1PI to BSC were identified. Three RCTs in
released vestigated the clinical efficacy
of A1PI compared to placebo. CT-measured lung density was the p
Act rimary o
and utcome in two RCTs, and
FEV1 was the primary outcome in one RCT. been
No significant differences between A1PI and placebo were identified in relation to mortality,
has
Health
exacerbation of COPD, hospitalisation due to COPD exacerbation, quality of life (SGRQ), respiratory
of
function (FEV1), exercise capacity (incremental shuttle walk test) or carbon monoxide diffusion
Information
capacity (DLCO). No relevant data was identified for dyspnoea.
of
The only statistically significant difference was observed for CT-measured lung density, which
document
favoured A1PI, however, the clinical significance of this difference is uncertain, as MCIDs for changes
in CT-lung density have not been established in the literature.
This Freedom
Department
The summary of findings (incor
the porating both benefits and harms) is shown in Table 1.
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
4
Page 18 of 218
FOI 5155 - Document 4
Table 1
Balance of clinical benefits and harms of A1PI relative to placebo as measured by the critical patient-
relevant outcomes in the key studies
Outcomes
Risk with
Risk with A1PI
Relative effect
Participants
Quality of
Comments
(units)
placebo
(95% CI)
(95% CI)
(studies)
evidence
Follow-up
(GRADE)
Uncertain due
Mortality
12 per 1,000
RR 0.35
180
34 per 1,000
⨁⨁⨁⨀
to low event
F/U 24 months
(2 to 78)
(0.05 to 2.27)
(1 RCT)
MODERATE
rate, RR subject
to error
Quality of life
MD 0.83 points
Direction
(SGRQ)
lower
248
favours
-
-
⨁⨁⨀⨀
placebo; not
F/U 24 to 30
(3.49 points lower
(2 RCT)
LOW
statistical y
months
to 1.82 points
higher)
significant
Annual
Higher reported RR
Direction
the
exacerbation
(1.26, 95% CI 0.92
favours
rate
-
-
257
to 1.74), MD (0.36,
⨁⨁⨁⨀
placebo; not
F/U 24 to 30
95% CI -0.44 to
(2 RCT)
MODERATE
statistical y
months
1.16) in A1PI group
significant
CT-measured
SMD 0.87 g/L
under
Direction
Care.
lung density
higher
304
(CTH)
-
-
⨁⨁⨁⨁
favours A1PI;
F/U 24 to 30
(0.31 higher to
(3 RCT)
HIGH
statistical y
months
1.42 higher)
significant
Mortality due to
No reported
1982 Aged
treatment-
deaths due to
related adverse
180
No treatment-related deaths reported
⨁⨁⨁⨀
released
treatment-
events
(1 RCT)
MODERATE
related adverse
Act and
F/U 24 months
events
Severe
Direction
been
adverse events
283 per 1,000
RR 0.83
257
341 per 1,000
⨁⨁⨁⨁
favours A1PI;
F/U 24 to 30
(195 to 406)
(0.57 to 1.19)
(2 RCTs)
HIGH
not statistically
months
Health
significant
has
Discontinuation
of
due to adverse
Direction
Information
events
10 per 1,000
RR 0.22
248
48 per 1,000
⨁⨁⨁⨀
favours A1PI;
not statistically
F/U 24 to 30
(2 to 62)
(0.04 to 1.30)
(2 RCTs)
MODERATE
of
significant
months
Hospitalisation
due to adverse
document
497
events
Median rate 1.4% (range 0.0% to 14.3%)
(4 observational ⨁⨁⨀⨀
-
F/U 3 to 6
studies)
LOW
Department
years
This Freedom
Abbreviations:
F/U = follow-up,
MD = mean dif erence,
RR = relative risk,
SGRQ = St George’s Respiratory Questionnaire,
SMD =
standardised mean dif erence. the
GRADE Working Group grades of evidence (Guyatt et al., 2013)
⨁⨁⨁⨁
High quality: We are very confident that the true ef ect lies close to that of the estimate of effect.
by
⨁⨁⨁⨀
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the
effect, but there is a possibility that it is substantially dif erent.
⨁⨁⨀⨀
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially dif erent from the estimate of
the effect.
⨁⨀⨀⨀
Very low quality: We have very lit le confidence in the effect estimate: The true effect is likely to be substantially dif erent from
the estimate of effect.
On the basis of the benefits and harms reported in the evidence base (summarised above),
it is
suggested that, relative to BSC, A1PI has inferior safety and uncertain effectiveness. This
conclusion is predicated on the understanding that the intervention poses an additional risk of
adverse events in addition to conservative management, noting that most adverse events associated
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
5
Page 19 of 218
FOI 5155 - Document 4
with the intervention were mild, and severe adverse events were not significantly different across
treatment and placebo arms in the RCTs.
Relative to placebo, there were no important differences in
safety outcomes.
TRANSLATION ISSUES
Three key issues arise in translating the evidence provided in Section B to an economic model
presented in Section D. The first, relates to the applicability of the populations in the pivotal RAPID
trial to clinical practice in Australia; the second, concerns selection of utilities; and the third, involves
extrapolation of trial evidence beyond the maximum follow-up of RAPID. The key uncertainty is that
the RAPID trial only had a maximum follow-up of four years on the intervention arm, however, AATD
is a chronic condition for which AT is likely to provide longer-term clinical impacts. A b
the ase four-year
trial analysis and a stepped analysis, where the model timeframe is extrapolated over a lifetime, are
included in the economic analysis. Patients are assumed to stay on no decline, slow and rapid
under
decline tracks for the remaining 26 years of the projection after the trial period. Annual mo
Care. rtality
(CTH)
during the RAPID trial is used for the trial period, after which extrapolation of survival is undertaken
using parametric models fitted to the UK registry data using analysis undertaken by CSL Behring.
1982 Aged
Given that the majority of clinical benefits are estimated during the extrapolated period, for which
released
there is no trial evidence, considerable uncertainty exists around economic results.
Act and
ECONOMIC EVALUATION
been
A cost-utility analysis was undertaken to determine the value
Health of AT in addition to optimal
has
pharmacological treatment and supportive care (bes
of t supportive care). A summary of the key
characteristics of the economic evaluation are provided be
Information low (Table 2), for more detail see Table
62.
of
Table 2
Summary of the economic evaluation
document
Perspective
This economic evaluation was conducted from the perspective of the Australian health
system. It includes resourc
Department e use supported by government and patients, along with
This
health out
Freedom comes applicable to the treatment of patients with emphysema due to A1PI
deficiency.
the
Intervention
Augmentation therapy in addition to optimal pharmacological treatment and supportive
by care.
Comparator
Best Supportive Care. Optimal pharmacological treatment and supportive care
Type of economic evaluation
Cost-utility analysis
Sources of evidence
RAPID study, RAPID-OLE study, UK Registry data
Time horizon
30-year time horizon in the base case
Sensitivity analyses include a time horizon of 20 years and 40 years
Outcomes
Quality-adjusted life years (QALY)/ life-years gained
Methods used to generate
Cohort expected value analysis
results
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
6
Page 20 of 218
FOI 5155 - Document 4
Health states
1.
FEV1≥50% predicted, no lung density decline
2.
FEV1≥50% predicted, slow lung density decline
3.
FEV1≥50% predicted, rapid lung density decline
4.
FEV1<50% predicted, no lung density decline
5.
FEV1<50% predicted, slow lung density decline
6.
FEV1<50% predicted, rapid lung density decline
7.
Lung transplant
8.
Dead
Cycle length
1 year
Discount rate
5% used for base and 3.5% and 7% sensitivity analyses
Software packages used
Microsoft Excel 2010
Abbreviations:
MBS = Medicare Benefits Schedule;
QALY = quality adjusted life year.
Only a limited number of economics studies relating to AT cost-effectiveness were identified in the
the
literature. Two studies related resource use to expected life gain using USA registry data. High
incremental expected survival of more than seven years in non-smokers resulted in AT appearing
relatively cost-effective. Gildea et al. (2003) developed a model where health s
under tates were stratified
(CTH) Care.
by COPD severity using FEV1 defined ranges. This approach is also adopted in COPD modelling more
broadly. RAPID was powered to detect changes in CT-scanned lung density. Correspondingly, the
patient level data and model developed by CSL Behring defined health states
1982 by FEV
Aged 1 predicted and
CT lung density decline tracks. This approach is followed in this assess
released ment.
Act and
The incremental cost and the incremental effectiveness of adding AT to BSC as an intervention
been
relative to BSC as a comparator are presented in Table 79, and briefly in Table 3. The incremental
cost-effectiveness ratio is presented as the incremental cost of achieving an additional QALY. It has
has
Health
been found that the lifetime ICER is s47(1)(b) per QALY and for the trial period is s47(1)(b) . It is
of
evident that most benefits accrue after the RAPID trial p
Information eriod, which is not based on clinical
evidence.
of
Table 3
Incremental Cost Effectiveness Ratio (1,000-patient cohort)
document
Cost
Incremental Effectiveness Incremental
ICER
cost
(QALYs)
effectiveness
This Freedom
Department
Trial period
the
A1PI Augmentation Therapy
s47(1)(b)
3,018.2
170.3
s47(1)(b)
Best Supportive Care
18,531,803
2,847.9
by
Lifetime
A1PI Augmentation Therapy
s47(1)(b)
6,010.6
1,351.5
s47(1)
(b)
Best Supportive Care
37,389,939
4,659.1
Abbreviations:
A1PI = alpha-1 proteinase inhibitor;
ICER = incremental cost effectiveness ratio;
QALY = quality-adjusted life year.
The assumption about the price paid for the AT product is the key driver of model results (Table 4).
The base cost of AT assumes a price per 1,000 ml of s47(1)
s47(1)
s47(1)
(b)
. This varies from (b)
to (b)
per 1,000ml
vial. The estimated ICER varies considerably from s47(1)(b)
to s47(1)(b)
per QALY. The transitions
assumed between no, slow and rapid decline patient states in the RAPID trial period also have a
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
7
Page 21 of 218
FOI 5155 - Document 4
large impact on the estimated ICER. CSL Behring provided confidential trial data at the patient level
that tracks the proportion of patients on the AT and BSC arms over the first two years for BSC and
four years for AT. Given that large proportions of patients are estimated to transition to the FEV1<50
rapid-decline group, the choice of parametric model for the purpose of estimating annual mortality
for this health state is important.
Table 4
Drivers of the economic model
Description
Method/Value
Impact
The average dosing for AT is taken from the RAPID The base cost of AT assumes a price per 1,000
s47(1)
s47(1)
s47(1)
Cost of the AT
trial and applied to an average weight of 75.9 kg.
ml ((b) ). This varies from (b) to (b) per
product
The number of vials (rounded to a whole number) is 1,000ml vial. The estimated ICER varies
multiplied by average, high and low AT product
considerably between s47(1)(b) and s47(1)(b)
prices.
per QALY.
the
Transition between There were considerable dif erences in transition
A higher number of patients move to the
FEV
FEV1<50 decline states on the BSC arm in
1 and CT
between health states for the AT and BSC arms in
density decline
the RAPID trials. The economic model assumes
RAPID. Movement during the trial period drives
under
during RAPID drives movement to no, slow and rapid decline tracks
economic results. Al owing transition between
(CTH) Care.
clinical benefit
during the trial period is sustained for a lifetime.
no, slow and rapid tracks after 4 years has
limited impact on the estimated ICER.
In most cases the Gompertz model is the best fit
The specification of the FEV<50 rapid-decline
Aged
Selection of
model to extrapolate survival and this model is used model had the lar
1982 gest impact on the estimated
extrapolation model across all non-transplant states. The model is varied ICER. The use of Lognormal, Generalised
released
for the FEV
as part of sensitivity analyses that included use of
1<50
Gamma and Weibull models resulted in the
Act and
rapid-decline group the Log-logistic, Lognormal, Weibull, Exponential
ICER being 10% more cost effective, while use
survival
and Generalised Gamma specifications. Large
numbers of patients transition to this state during the of the Exponential model resulted in a 10%
been
trial period, particularly on the BSC arm.
decrease in cost effectiveness.
Disease management costs in many reviewed Health
COPD economic models were an aggr
has egate of This variation has limited impact as economic
of
Disease
maintenance and acute care costs during flare ups. results are governed by AT product costs. The
management costs The frequency of flare ups was not explicitly
proportion of severe COPD patients who are
Information
for COPD
modelled in this assessment. The Thomas et al.
very severe, assumed to be 74% in the base
2014 analysis included acut
of e care proportions for cases, also varied. Similarly, this scenario had
each state. They are varied by 20% for each COPD limited impact on the estimated ICER.
state.
document
Abbreviations:
AT = augmentation therapy,
BSC = best supportive care,
COPD = chronic obstructive pulmonary disease,
CT =
computed tomography,
FEV1 = forced expiratory volume in 1 second,
ICER = incremental cost effectiveness ratio.
Department
E
This
STIMATED EXTENT OF USE AND F
Freedom
INANCIAL IMPLICATIONS
the
The financial impact of the potential listing of A1PI AT is calculated using an epidemiological
by
approach over a six-year period, based on an estimate of the number of patients eligible for
treatment. The financial impact of AT on the NBA is summarised in Table 5. The estimated cost is
presented over the six-year costing proposal period and is based on a s47(1)
(b)
uptake rate for AT by
2023. Uptake begins at s47(1)
s47(1)
(b)
and increases by (b) per year. The cost to the NBA for the total AT
market is estimated to be s47(1)(b)
in 2019, increasing to s47(1)(b)
in 2023.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
8
Page 22 of 218
FOI 5155 - Document 4
Table 5
Total costs to the NBA associated with AT
2019
2020
2021
2022
2023
AT-eligible patients
s47(1)(b)
% uptake of AT
AT patients across Australia
Average weight (kg)
76
76
76
76
76
Recommended dose (mg/kg body weight)
60
60
60
60
60
Grams of AT per patient per week
4554
4554
4554
4554
4554
Vials per patient per week
5
5
5
5
5
Adherence
94%
94%
94%
94%
94%
Number of vials across Australia
s47(1)(b)
Cost per 1,000ml vial ($)
Cost per patient per year ($)
the
Total cost of AT $
Abbreviations:
AT = augmentation therapy.
A key uncertainty is the price of AT. Variations in price have a large impact o
under n both financial and
(CTH) Care.
economic attractiveness because of the large contribution of the AT product itself to overall
resource in the economic model. The proposed price of PROLASTIN-C is s47(1)
(b)
per 1,000ml vial and
ZEMAIRA
Aged
s47(1)
s47(1)
s47(1)
s47(1)
1982
(b)
. An average price of (b)
is included, with (b)
and (b)
used as high and low
bounds in sensitivity analyses. Varying the prevalence proportions
released by 10% has a lesser financial
Act and
impact. Uptake rate also has an impact. A decrease in year 2022 uptake from s47(1)
s47(1)
(b)
to (b) results in a
s47(1)(b) budget requirement in that year. been
CONSUMER IMPACT SUMMARY has
Health
of
Six associations provided targeted feedback, and one individual provided non-targeted feedback on
Information
this consultation. All respondents using the feedback form ‘strongly agreed’ with the clinical claim
of
made by the applicant.
document
OTHER RELEVANT CONSIDERATIONS
This
Department
The accessibility of A1PI to rural an
Freedom d remote patients is potentially limited owing to distance from
specialist centres, respiratory p
the hysicians and temperature-controlled transport. Training required to
self-administer A1PI and the eligibility of lung transplant patients and current smokers for A1PI
by
therapy needs to be addressed.
A1PI meets three of the four criteria warranting rule of rescue. It is unclear whether the proposed
service provides worthwhile clinical improvement. Owing to the rarity of A1PI deficiency, clinical
trials are often under-powered to detect statistical differences in outcomes such as quality of life
(QoL) and mortality. Rather, studies use lung CT densitometry, an outcome correlative to markers of
lung health and mortality, to infer clinical efficacy. When this is taken into consideration with the
results from the trials listed in section B it is unclear whether A1PI provides worthwhile clinical
improvement.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
9
Page 23 of 218
FOI 5155 - Document 4
SECTION A
CONTEXT
This contracted assessment of purified human A1PI for the treatment of Alpha-1 anti-trypsin
deficiency (AATD) leading to chronic obstructive pulmonary disease (COPD) is intended for the
Medical Services Advisory Committee (MSAC). MSAC evaluates new and existing health technologies
and procedures for which funding is sought under the Medicare Benefits Schedule (MBS) in terms of
safety, effectiveness and cost effectiveness, while taking into account other issues such as access
and equity. MSAC adopts an evidence-based approach to its assessments, based on reviews of the
scientific literature and other information sources, including clinical expertise. This application was
received on behalf of the National Blood Authority, for listing of A1PI on the NPL for blood products.
the
Research and Evaluation, incorporating ASERNIP-S of the Royal Australasian College of Surgeons has
been commissioned by the Australian Government Department of Health to conduct a systematic
under
literature review and economic evaluation of purified human A1PI for the treatme
(CTH) nt of alp
Care. ha1-
proteinase inhibitor deficiency, leading to COPD. This assessment has been undertaken in order to
inform MSAC’s decision-making regarding whether the proposed medical service should be publicly
1982 Aged
funded. Appendix A provides a list of the people involved in the development of this assessment
released
report.
Act and
The proposed use of A1PI in Australian clinical practice was outlined in an MSAC application that was
been
released in a targeted consultation on 2 February 2018. The subsequent PICO Confirmation was
Health
presented to the PICO Advisory Sub-Commit
has tee (PASC) and ratified on 7 June 2018.
of
A.1.
I
Information
TEMS IN THE AGREED PICO CONFIRMATION
of
This contracted assessment largely conforms to the PICO elements that were pre-specified in the
PASC-ratified PICO Confirmation. Th
document ere are two key deviations from the proposed PICO criteria:
1. The proposed population was focussed on
Department the primary indication of severe A1PI deficiency
This Freedom
(serum levels ≤11 μM) and emphysema with FEV1 <80%. Evidence for effectiveness was
the
limited to this population group. Safety data investigating adverse events of A1PI infusion
by
were broadened slightly in order to capture adverse events associated with AT.
2. No studies comparing AT to optimal pharmacological treatment and supportive care were
identified. In lieu of this, placebo-controlled trials were included.
A.2.
PROPOSED MEDICAL SERVICE
The proposed medical service is for lifelong intravenous blood augmentation via weekly infusions of
purified human A1PI. This is the first time that purified human A1PI has been assessed by MSAC.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
10
Page 24 of 218
FOI 5155 - Document 4
AT is an additive intervention that will be given in addition to BSC for patients with emphysema
(Ranes and Stoller 2005). Therapeutic concentrations of A1PI are prepared from the blood of plasma
donors. The product is presented as a sterile lyophilised powder in a 1g vial. It needs to be
reconstituted in 20 mL of water for intravenous administration. Treatment takes fifteen minutes and
is conducted in an outpatient hospital or clinic setting in the first instance. Patients may administer
the therapy at home after receiving adequate training and when deemed appropriate by the treating
specialist.
There are currently no established doses or regimens for A1PI augmentation therapy. Product
information for PROLASTIN-C recommends administering 60mg/kg of the drug intravenously once a
week (Therapeutic Goods Administration 2016). This dose and frequency was used in the RAPID and
the
EXACTLE RCTs (Chapman et al. 2015; Dirksen et al. 2009), however, the precise dose that confers the
greatest clinical efficacy is yet to be determined. Higher weekly doses of up to 120 mg/kg have been
evaluated for safety outcomes. The SPARK study was a multicentre RCT with four months follow-up.
under
Participants were treated with a weekly infusion of either 60mg/kg or 120mg/kg of PROLASTIN
Care. -C for
(CTH)
eight weeks, fol owed by a cross-over period. This study reported that the higher dose can be given
for at least eight weeks, being safe and well tolerated. It should, however, be studied for a longer
1982 Aged
period to ascertain effectiveness (Campos et al. 2013). In contrast, a pharmacokinetic case series
released
study of A1PI treatment found that, while safe, 120mg/kg every two weeks did not maintain
Act and
appropriate serum levels above 80mg/dL for the whole two weeks (Barker et al. 1997). An ongoing
trial, the SPARTA trial, aims to investigate the effica
been cy and safety of weekly 60mg/kg or 120mg/kg
doses of PROLASTIN-C versus placebo in a multicentre RCT with three years follow-up. The findings
Health
of this study are not yet published (Sorrels e
has t al. 2015).
Information
of
Access issues could exist in rural and remote areas of Australia where specialist care is not always
available to provide AT. Patients may o
of pt to move nearer to specialist centres to receive weekly AT,
however, this is not always an option.
document
PROLASTIN-C and Zemaira (marketed as Respreeza in Europe), are two AT products registered on the
Department
Australian Register
This of Therapeutic Goods (ARTG) in Australia. The two therapies consist of the same
Freedom
components with slightly different eligibility criteria (see Table 6). Both products are provided in a
the
pack containing: by
• 1 vial 1g lyophilised powder
• 1 vial 20 mL sterile water for injection
• 1 sterile filter needle
• 1 vented transfer device
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
11
Page 25 of 218
FOI 5155 - Document 4
Table 6
Approved augmentation therapies and their indications
Product
ARTG ID and details
PROLASTIN-C
ARTG ID 234553: indicated to increase serum A1PI levels in adults with congenital deficiency of alpha-1
anti-trypsin and with clinically significant emphysema (FEV1 less than 80%). The data for clinical
ef icacy of PROLASTIN-C is derived from changes in the biomarkers alpha-1 anti-protease level and CT
lung density. Ef icacy on FEV1 or patient relevant endpoints such as quality of life or pulmonary
exacerbations has not been established in randomised clinical trials. Clinical trials have only included
patients who were not smoking.
Zemaira
ARTG ID 273182: indicated for maintenance treatment, to slow the progression of emphysema in adults
with documented severe A1PI deficiency (A1PI less than 11 μM) and progressive lung disease. Patients
are to be under optimal pharmacologic and non-pharmacologic treatment.
Abbreviations:
ARTG = Australian Register of Therapeutic Goods,
FEV1 = forced expiratory volume in 1 second,
μM = micromolar.
EQUIVALENCE OF PROLASTIN-C AND ZEMAIRA
the
Two studies have investigated the bioequivalence of PROLASTIN-C and Zemaira to Prolastin (Table
7). PROLASTIN-C has been proven by a 24-week crossover study to have pharmacokinetic
under
equivalence and a comparable safety profile to Prolastin (Stocks et al. 2010b). Prolastin has been
(CTH) Care.
approved for use in the United States for over 35 years. Another study by the same group
demonstrated that the newer product, Zemaira, is bioequivalent to Prolastin by a 24-week double
1982 Aged
blind study with an open label extension (Stocks et al. 2006). Since both products are equivalent to
released
Prolastin, they are considered in this assessment to be equivalent to each other.
Act and
Table 7
Studies evaluating the biocompatability of A1PI therapies
been
Authors
Study
Location Description Description of Relevant outcomes Measurement Outcomes
Publication design Length of
Comparator
assessed
of outcomes
has
Health
Year
Evidence of follow- Intervention
and analysis
of
Study ID
level
up
Information
Stocks et al. RCT
United
Weeks 1 – Weeks 1 – 10
Primary outcome
T-test
Mean
2006
Cross
States
24 of Prolastin
• Trough serum
dif erence
over
24 weeks Zemaira
60mg/kg per
antigenic A1PI
from baseline
Level II
60mg/kg per week
levels
(Zemaira –
document
week
Weeks 11 – 24
Secondary outcome
Prolastin)
Zemaira
447.5nM
p =
• Adverse events
N = 30
60mg/kg per
Department
0.40
This
*analysi
Freedom s week
performed on
Zemaira vs
the 29 patients N = 14
Prolastin
121 vs 73 AE
by
2 vs 3 TAE
3 vs 10 SAE
0 vs 0 TSAE
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
12
Page 26 of 218
FOI 5155 - Document 4
Stocks et al. RCT
United
Weeks 1 - 8 Weeks 1 - 8
Primary outcome
ANOVA
Ratio of point
2010
Cross
States
PROLASTIN- Prolastin
• Pharmacokinetic Wilcoxon Rank estimates and
over
24 weeks C 60mg/kg 60mg/kg per
comparability:
test
90% CIs for
Level II
per week
week
AUC
AUC
0-7 days
0-7 days
Weeks 9 - 16 Weeks 9 - 16
Secondary outcome
PROLASTIN-
Prolastin
PROLASTIN-C
C vs Prolastin
• Adverse events
60mg/kg per 60mg/kg per
1.03 (0.97,
week
week
1.09) vs 0.98
Weeks 17 - Weeks 17 - 24
(0.95, 1.02)
24
PROLASTIN-C
PROLASTIN- 60mg/kg per
PROLASTIN-
C 60mg/kg week
C vs Prolastin
per week
11 vs 9 AE
N=12
0 vs 2 SAE
N = 12
0 vs 0 TAE
the PROLASTIN-
C weeks 17 -
24
under
11 AE and 0
(CTH) Care.
SAE in
Abbreviations:
A1PI = alpha-1 proteinase inhibitor,
AE = adverse event,
ANOVA = analysis of variance,
AUC = area under the curve,
CI
= confidence interval,
RCT = randomised controlled trial,
SAE = serious adverse event,
TAE = treatment-related adverse event,
TSAE =
treatment-related serious adverse event.
Aged
released 1982
CURRENT FUNDING ARRANGEMENTS
Act and
The proposed intervention is not currently reimbursed in Australia. AT is currently provided via out-
been
of-pocket payments, however, for many patients, the majority of whom are not working, the cost is
prohibitive for lifelong treatment. has
Health
of
A.3.
PROPOSAL FOR PUBLIC FUNDING
Information
of
No MBS item descriptor is required for this application. AT is proposed for reimbursement on the
NPL managed by the National Blood Authority. New blood and blood-related products reviewed by
document
the Jurisdictional Blood Committee may be referred to MSAC for evidence-based evaluation of the
safety, clinical effectiveness or cost-effectiveness. Th
Department is is the case with A1PI augmentation.
This Freedom
A.4.
P
the
ROPOSED POPULATION
by
The population to be considered in this assessment is ex- or never-smoking patients with
emphysema and severe A1PI deficiency. Severe A1PI deficiency is defined as serum levels below 11
μM (approximately 59 mg/dL) (Hatipoglu and Stoller 2016). Clinically, this deficiency manifests as
panacinar emphysema or hepatitis, cirrhosis, and/or hepatoma (Pharmacy and Therapeutics 2010).
Less commonly, vasculitis and panniculitis are observed (Pharmacy and Therapeutics 2010).
The chance of emphysema developing with A1PI deficiency increases across the PiMZ, PiSZ and PiZZ
phenotypes, with the most significant contributor being the PiZZ phenotype (de Serres and Blanco
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
13
Page 27 of 218
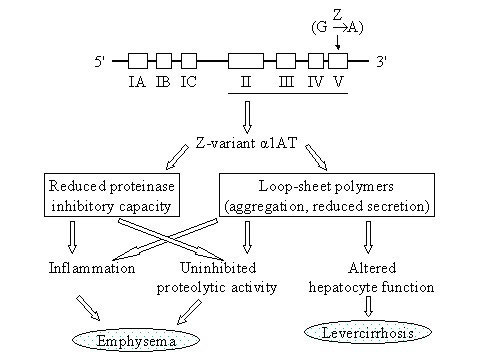
FOI 5155 - Document 4
2014). The assessed treatment is to be limited to patients with the PiZZ or nul phenotypes (see
Table 8 for detail on these phenotypes).
PATHOPHYSIOLOGY OF CONDITION
AATD is an inherited genetic condition that results in decreased circulating, and/or abnormally
functioning, A1PI protein. A1PI is predominantly synthesized by hepatocytes and released into the
bloodstream where it acts as a serine protease inhibitor, with neutrophil elastase being its primary
substrate (de Serres et al. 2003). A1PI deficiency, defined as ≤30% of normal serum levels, is known
to have a role in the development of liver disease and emphysema, and has been hypothesised to be
part of pathological processes underlying a range of health conditions.
the
The consequences of A1PI deficiency for lung and liver function occur via different pathways. In the
lungs, neutrophil elastase, which has an important role in fighting infection, is normally bound and
inactivated by A1PI. Low levels of A1PI mean that the enzymatic activity of neutrophil elastase goes
under
unchecked and ultimately its detrimental impact on elastin compromises the bronch
(CTH) ia and al
Care. veoli.
Conversely, liver damage occurs when the A1PI protein forms polymers that accumulate within
hepatocytes, leading to scarring, inflammation or malignancy. Figure 1 shows a simplified schematic
1982 Aged
of the mechanisms underlying disease associated with the most prevalent allele causing A1PI
released
deficiency (Fregonese and Stolk 2008).
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Figure 1
Simplified schematic of the pathway to lung and liver disease associated with A1P1 deficiency
(Fregonese and Stolk 2008)
A1PI production is specified by a pair of co-dominant alleles on the SERAPINA1 gene, of which the
PiMM (protease inhibitor, homozygote M) is the most common and normal functioning state.
Individuals with only one abnormal gene (e.g. PiMZ or PiMS) may have reduced production of A1PI
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
14
Page 28 of 218
FOI 5155 - Document 4
but are often asymptomatic and are considered carriers. Genetic variants with at least 100 alleles
have been described. The most prevalent deficiency-causing allele is the Z allele, of which the PiZZ
state is amongst the most severe manifestations of deficiency (Brode et al. 2012). PiSZ and other
rare variants also contribute to the burden of disease attributable to A1PI deficiency (Häggblom et
al. 2015). In rare cases, patients with a PiNul /Nul phenotype do not produce any A1PI.
INCIDENCE IN AUSTRALIA
Serum A1PI levels associated with selected variants, including those contributing to early onset
emphysema, are shown in Table 8 (adapted from Hatipoglu and Stoller 2016). Prevalence data from
Australia is limited, however, de Serres et al. (2003) reported gene frequencies per 1,000 people
from a range of cohort studies conducted in various populations in Australia. De Serres et al. (2003)
the
reported that the estimated prevalence of carriers of deficiency alleles in the Australian population
is 1 in 8.9 individuals; the majority of whom are carriers. For PiSZ the prevalence was estimated to
be 1 in 841 and for PiZZ it is estimated at 1 in 5,584. It is the PiZZ allele that contributes to the
under
(CTH) Care.
greatest burden of lung disease in the A1PI deficient population.
Lung manifestations of disease present in adulthood and early symptoms are common to a range of
1982 Aged
conditions, thus the number of patients with a diagnosis is likely to be an under-estimate of the true
released
prevalence of the condition. Changes in the reported prevalence estimates are only likely to affect
Act and
uptake. However, if genetic or phenotype testing for A1PI deficiency becomes more common for
COPD patients or family members of known A1PI defi
been cient patients.
Health
Further, it was noted by PASC that not al p
has eople with PiZZ A1PI deficiency wil go on to develop
severe emphysema. Based on estimations made by th
of e commercial sponsors, the incidence of
Information
people meeting the criteria for treatment with A1PI in Australia was s47(1
)(b)
in 2018. More precise
of
estimates of the prevalence of the condition and potential uptake are reported in Section E.1.
Considering treatment is expected to be lifelong and is not curative, the number of patients being
document
treated will have a cumulative increase over time.
Department
Table 8
Serum
This
A1PI levels associated with normal and SZ or ZZ allele variations known to increase the risk
Freedom
of emphysema (Hatipoglu and Stol er 2016)
the
Alleles Impact
Serum A1PI levels
Genetic prevalence in the Australian
by
Mg/dL (Mean [5th–95th
population (de Serres et al. 2003)**
Percentile])
MM
Normal
147 (102–254)
Not applicable
MS or
Carriers, usually asymptomatic
125 (86–218)
1 in 12
MZ
90 (62–151)
1 in 40
SS
Slightly increased emphysema
95 (43–154)
1 in 507
risk, mildly symptomatic or
asymptomatic
SZ
Individuals produce less A1PI
62 (33–108)
1 in 841
than normal and have an
increased risk of emphysema
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
15
Page 29 of 218
FOI 5155 - Document 4
Alleles Impact
Serum A1PI levels
Genetic prevalence in the Australian
Mg/dL (Mean [5th–95th
population (de Serres et al. 2003)**
Percentile])
ZZ*
Most severely affected ,
≤29 (≤29–52)
1 in 5,584
individuals have a greatly
increased risk of emphysema
and liver disease
Null
Very rare, no A1PI produced
0
Very rare, cannot be estimated
Abbreviation:
A1PI = alpha-1 proteinase inhibitor.
*It has been estimated that the number of individuals with the ZZ form in Australia is 4,126 (between 2,894–5,695)(Blanco et al. 2017).
**Genetic prevalence (95% confidence interval) per 1,000 for the PiS allele is 44.4 (40.7–48.5); for the PiZ allele it is 13.4 (11.4–15.7).
A.5.
COMPARATOR DETAILS
There are currently no active comparators for AT that modify the progression of e
the mphysema or
COPD in patients with AATD. The comparator for this intervention is best supportive care, which is
aimed primarily at symptom management and control of COPD exacerbations and respiratory
under
infections. Strategies for the management of stable COPD are provided in the Australian and New
(CTH) Care.
Zealand guidelines for the diagnosis and treatment of COPD (Yang et al. 2017) as follows:
NON-PHARMACEUTICAL STRATEGIES
Aged
released 1982
Pulmonary rehabilitation and physical activity are strongly evidenced to be effective in optimising
Act and
function (Yang et al. 2017). Pulmonary rehabilitation includes supervised exercise training and can
be given in conjunction with any number of the fol
been owing: behaviour change, nutritional advice or
psychosocial support.
has
Health
P
of
HARMACOLOGICAL STRATEGIES
Information
Inhaled medications are the primary pharmacological strategy for managing COPD (Yang et al. 2017).
of
A stepwise approach is recommended for taking inhaled medicines, irrespective of severity, until
adequate control is reached (Lung Foundation Australia). The aim of pharmacological strategies is to
document
reduce symptoms, prevent exacerbations, and improve health status by targeting the
pathophysiology of the disease.
This Freedom
Department
the
Apart from inhaled medications, corticosteroids and antibiotics can be recommended. Oral
corticosteroids hasten re
by solution of exacerbations and reduce the likelihood of relapse. For purulent
sputum, antibiotics may also be recommended to address typical and atypical organisms.
Furthermore, comorbidities that often accompany COPD, the main ones being anxiety and
depression, increase hospitalisation and need to be managed. Osteoporotic fractures are also a
common problem in patients with COPD, hence bone mineral density testing is important for
prevention and monitoring. COPD and its resulting hypoxaemia are known to lead to pulmonary
hypertension and right heart failure, especially when occurring with sleep apnoea. When this is
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
16
Page 30 of 218
FOI 5155 - Document 4
suspected clinically, arterial blood gas or a sleep study should be conducted, leading to oxygen
therapy or continuous positive airway pressure.
PREVENTION OF DETERIORATION
Behavioural change is also recommended (Yang et al. 2017). In the hope of preventing deterioration,
patients are recommended to cease cigarette smoking (of utmost importance), reduce alcohol
consumption, increase physical activity, and avoid environmental irritants.
Another helpful approach is vaccination against influenza and pneumococcal, as it reduces
exacerbations due to influenza and pneumococcal in high-risk seasons. When used together there is
an additional benefit.
the
Long-term use of supplemental oxygen assists correction of severe hypoxaemia and might also
improve survival. Use of supplemental oxygen for longer periods has been reported to have greater
under
benefits. For all COPD patients, ambulatory oxygen may be of benefit when blood is desaturated due
(CTH) Care.
to exertion.
END STAGE STRATEGIES
Aged
released 1982
Lung transplantation or lung volume reduction, either by surgery or bronchoscopically, might be
Act and
required for patients with very severe disease (Yang et al. 2017). Only certain patients may be
considered appropriate for lung volume reductio
been n, such as those with severe emphysema,
hyperinflation and ongoing symptoms despite best management and pulmonary rehabilitation.
Health
Likewise, patients considered for lung tran
has splantation would be those suffering severe functional
of
impairment and airflow obstruction not appropriately managed by other strategies. PASC noted that
Information
lung transplantation is not curative and transplant recipients would still be in need of A1PI
of
supplementation to prevent gradual deterioration of the lungs.
document
A.6.
CLINICAL MANAGEMENT ALGORITHM
Department
The current and pr
This oposed clinical management algorithms are presented in Figure 2 and Figure 3,
Freedom
respectively. Patients are cu
the rrently managed with BSC, including pharmacological symptom
management and non-pharmacological strategies mentioned in Section A.5. Current treatment
by
strategies are primarily aimed at alleviating COPD symptoms, and are not disease modifying.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
17
Page 31 of 218
FOI 5155 - Document 4
Patients with
emphysema and FEV1
<80%
Investigated for A1-
PI deficiency with
serum levels and
genotyping
the
under
Optimal
Optimal
(CTH) Care.
pharmacological
Documented A1-PI
pharmacological
therapy and best
Yes
deficiency
No
therapy and best
supportive care
supportive care
1982 Aged
Figure 2
Current clinical management algorithm for patients with emphysem
released
a and FEV1 <80%
Act and
been
AT is an additive intervention, which will be given in addition to BSC for patients with emphysema
(Ranes and Stoller 2005). The main difference in the current and proposed treatment pathways is
has
Health
the necessity for patients to stop smoking in order for AT to be effective.
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
18
Page 32 of 218
FOI 5155 - Document 4
Patients with
emphysema and FEV1 <
80%
Investigated for A1-PI
deficiency with serum
levels and genotyping
Optimal
the
pharmacological
Currently
yes
therapy and
smoking
assistance with
smoking cessation
under
(CTH) Care.
No
Once quit smoking
1982 Aged
for minimum 6
months can re
released -join
treatment*
Act and
been
Optimal
Deficiency is
pharmacological
Health
≤11 μM
No
has
therapy and best
of supportive care
Information
of Yes
document
This
Department
Augmentation therapy with
Freedom
Prolastin-C or Zameira in addition
the
to optimal pharmacological
treatment and supportive care
by
*Patients should be monitored for
failure to quit smoking, and if relapse
occurs they will lose access to the
intervention
Figure 3
Proposed clinical management algorithm for patients with emphysema and FEV1 <80%
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
19
Page 33 of 218
FOI 5155 - Document 4
A.7.
KEY DIFFERENCES IN THE DELIVERY OF THE PROPOSED MEDICAL SERVICE AND THE
MAIN COMPARATOR
The key difference is the outcome that the intervention and comparator attempt to achieve. The
comparator of BSC includes a number of approaches aiming to address the symptoms of the
condition, optimise function, and prevent deterioration. The proposed service is intended to be used
in combination with BSC, and is proposed to slow progression of the disease.
A.8.
CLINICAL CLAIM
The applicant claims that A1PI augmentation will slow the progression of A1PI deficiency and its
the
accompanying symptoms, and is superior to currently available treatments forming the comparator
intervention.
under
A.9.
SUMMARY OF THE PICO
(CTH) Care.
The guiding framework of a PICO Confirmation is recommended by MSAC for each assessment. The
1982 Aged
PICO Confirmation describes current clinical practice and reflects the likely future practice with the
released
proposed medical service.
Act and
The Population, Intervention, Comparator and Outcomes (PICO) that were pre-specified to guide the
been
systematic literature review are presented in Box 1 and Box 2.
Health
Box 1
Criteria for identifying and selecting
has
studies to determine the safety of purified human A1PI for the
treatment of A1PI deficiency, leading to COPD
Information
of
Selection criteria
Description
Population
A1PI deficiency of
Intervention
AT with any A1PI product (PROLASTIN-C, Zemaira, or other)
Comparator
Best supportive c
document are for COPD
Outcomes
Critical for decision making:
• Mortality due to adverse ev
Department ents
This Freedom
• Severe adverse events
•
the Discontinuation due to adverse events
• Hospitalisation due to adverse events
by Important, but not critical for decision making:
• Treatment-related adverse event
• Any adverse event
• Infection from treatment
• Dyspnoea
Systematic review
What is the safety of purified human A1PI for the treatment of alpha1-proteinase inhibitor
question
deficiency, leading to COPD?
Abbreviations:
A1PI = alpha-1 proteinase inhibitor,
AT = augmentation therapy;
COPD = chronic obstructive pulmonary disorder.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
20
Page 34 of 218
FOI 5155 - Document 4
Box 2
Criteria for identifying and selecting studies to determine the effectiveness of purified human A1PI
for the treatment of A1PI deficiency, leading to COPD
Selection criteria
Description
Population
A1PI deficiency
Intervention
AT with either Prolastin or Zemaira
Comparator
Best supportive care for COPD
Outcomes
Critical for decision making:
• Mortality, including deaths from respiratory failure
• Patient quality of life (measured by validated tool for COPD or respiratory impairment)
• Number of exacerbations and hospitalisations associated with emphysema
• Surrogate measures: CT-measured lung density, carbon monoxide transfer or dif usion
capacity (DLCO)
Important, but not critical for decision making:
• The BODE index- body mass index, airflow obstruction, dyspnoea and ex
the ercise index
• Changes in exercise capacity (per 6-minute walking test)
• Dyspnoea (measured with a validated tool e.g. baseline dyspnoea index, transition
dyspnoea index)
under
• Respiratory function measured by spirometry (FEV1) and FEV1/Forced vital capacity
(CTH) Care.
(FVC) ratio
Systematic review What is the efficacy of purified human A1PI for the treatment of alpha1-proteinase inhibitor
question
deficiency, leading to COPD?
1982 Aged
Abbreviations:
A1PI = alpha-1 proteinase inhibitor,
AT = augmentation therapy;
BODE = BMI, obstruction, dyspnea, exercise capacity;
COPD = chronic obstructive pulmonary disorder,
CT =computed tomography,
DLCO = dif using capacity for carbon monoxide,
FEV
released
1 =
forced expiratory volume in 1 second,
FVC =forced vital capacity.
Act and
A.10.
CONSUMER IMPACT STATEMENT
been
Six associations provided targeted feedback and one individual provided non-targeted feedback on
has
Health
this consultation. All respondents using the feedback form ‘strongly agreed’ with the clinical claim
of
made by the applicant. Respondents have been de-identifie
Information d for the purpose of this report.
of
Australia’s foundation for the affected organ supports the assessment of this intervention. In parallel
to this assessment it is forming a working group and a position statement or guideline on AT. This
document
statement wil be updated in response to the results of the assessment. The quote below perhaps
best outlines the reasons for this assessment an
Department d confirms the current state of AT, including
This Freedom
uncertainties around the dosing strategy and cost effectiveness:
the
“It is noted that the optimal dosing regimen has not yet been determined, and the cost-effectiveness
by
of AT is not known. On the balance of the evidence to date and methodological considerations, AT
with this current treatment approach is not yet recommended, and results from additional
randomised controlled trials (RCTs) underway and other analyses are awaited.”
A binational health promotion charity noted the low availability of data due to the rare nature of this
condition. It noted that the efficacy of AT is only supported by clinical consensus at this stage.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
21
Page 35 of 218
FOI 5155 - Document 4
This group strongly suggests that strict access to augmentation should be provided, with criteria
such as: “prescription through a respiratory specialist, with further assessment of patient lung
function within an accredited respiratory function laboratory.”
The Australian foundation supporting patients with Alpha-1 provided evidence that it believes shows
a significant increase in life expectancy for patients treated with AT. Subject to funding, it wishes to
establish an Australian register for Alpha-1 patients to better understand the epidemiology of the
condition.
The foundation provided two letters to the National Blood authority (the applicant) regarding the
need for AT for AATD patients and supporting efforts to secure public reimbursement. It also
provided an article from the foundation’s newsletter introducing the AlphaNet study and reporting
the
the unpublished results, and a conference poster on the AlphaNet study reporting survival analysis
on the three cohorts of A1PI patients receiving AT.
under Care.
In addition, the foundation provided seven written communications from patients, all e
(CTH) xpressing the
sentiment that the public funding of AT is critical to their ongoing disease management. The
foundation claims that these are merely examples put into writing of the kinds of contact it has been
1982 Aged
receiving from those affected by A1PI for the past 12 years.
released
Act and
Another personal account has been received directly from a patient/private individual to the effect
that his/her life situation could have been greatly imp
been roved if AT were available.
The manufacturers of the two products prov
has ided feedback as well as
Health information for the application.
Overal , much consideration was put into the responses
of , with most respondents providing additional
Information
sources with the response form. of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
22
Page 36 of 218
FOI 5155 - Document 4
SECTION B
CLINICAL EVALUATION
B.1.
LITERATURE SOURCES AND SEARCH STRATEGIES
To identify relevant studies, the medical literature was searched in Embase on 23 May 2018, and in
PubMed and The Cochrane Library on 24 May 2018. No date limit was used in these searches. Search
terms were aimed at retrieving information on all A1PI augmentation therapies.
Attempts were also made to source unpublished or grey literature from New York Academy of
Medicine Grey Literature Report, CEA Registry, National Information Centre of Health Services
the
Research and Health Care Technology (NICHSR), National Library of Medicine Health
Services/Technology Assessment Texts (HSTAT), EuroScan International Network, National Institute
for Health and Care Excellence (NICE), NHS National Institute for Health Research (NIHR) — including
under
HTA programme and Online Mendelian Inheritance in Man, as well as the manufacturer
Care.s and
(CTH)
specialty societies: Grifols, CSL Behring and SHIRE, National Blood Authority, Lung Foundation
Australia, the Thoracic Society of Australia and New Zealand, and Alpha-1 Foundation.
Aged
released 1982
Databases, sources, search terms and outcomes for each database are described in Appendix B.
Act and
B.2.
RESULTS OF LITERATURE SEARCH
been
A PRISMA flowchart (Figure 4) provides a graphic depiction of the results of the literature search and
has
Health
the application of the study selection criteria (listed in Box 1 and Box 2) (Liberati et al. 2009).
Information
of
Studies were selected independently by two reviewers (TV, AS). Disagreements regarding study
of
selection were resolved by discussion between the two reviewers.
document
Studies that could not be retrieved or that met the inclusion criteria but contained insufficient or
inadequate data for inclusion are listed as Excluded Studies in Appendix D. All other studies that met
This
Department
the inclusion criteria are listed in A
Freedom ppendix C. A list of trials that appeared to be relevant but were
excluded on ful -text review is p
the rovided in Appendix E.
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
23
Page 37 of 218
FOI 5155 - Document 4
Records identified through
Additional records identified
database searches
through other sources
(n = 7525)
(n = 1)
Identification
Duplicates removed
(n = 2679)
Records screened by title and
abstract
(n = 4847)
Screening
Studies excluded due to: 4723
the
Incorrect study design (n = 237)
Incorrect publication type (n = 998)
Not in English (n = 596)
Incorrect population (n = 1887)
under Care.
Incorrect intervention (n = 707)
(CTH)
Full-text articles assessed for
Incorrect outcome (n = 298)
eligibility
(n = 124)
Aged
Eligibility
1982
Studies excluded due to: 102
released Incorrect study design (n = 45)
Act and
Incorrect population (n = 8)
Incorrect intervention (n = 6)
been
Incorrect comparator (n = 1)
Studies included in qualitative
Incorrect outcome (n = 42)
has
Health
synthesis
(n = 22)*
Information
of
Included
of
Included for safety:
RCTs (n = 3)
Included for effectiveness:
NRCTs (n = 1)
RCTs (n = 3)
document
Single arm (n = 13)
NRCTs (n = 7)
This Freedom
Department
Figure 4
Summary of the process used to identify and select studies for the assessment
the
*Three RCTs and two single arm (NRCT) studies were included for both safety and effectiveness
analysis.
by
Table 9 lists the included randomised controlled trials (RCTs) and published reports based on each
trial. A profile of each included study is given in Appendix C. Appendix C This study profile describes
the authors, study ID, publication year, study design and quality (level of evidence and risk of bias),
length of patient follow-up, study population characteristics, description of the intervention,
description of the comparator, relevant outcomes assessed, and measurement of outcomes and
analysis. Study characteristics are also summarised in a shorter format in Section B.4.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
24
Page 38 of 218
FOI 5155 - Document 4
In addition to the RCTs, 14 observational studies were included to evaluate the safety of A1PI.
Characteristics of these studies are outlined in Section B.4, and Appendix B.
Table 9
Trials (and associated data) presented in the assessment report
Trial
Reports
Effectiveness
RCTs
RAPID
Chapman K, Burdon J, Pi tulainen E, et al. 2015. Intravenous augmentation treatment and lung density in
severe A1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial,
Lancet;
386(9991); 360-368.
McElvaney N, Burdon J, Holmes M, et al. 2017. Long-term efficacy and safety of A1 proteinase inhibitor
treatment for emphysema caused by severe A1 antitrypsin deficiency: an open-label extension trial
the
(RAPID-OLE),
Lancet Respir Med, 2017, 5(1): 51-60.
NCT00261833, Zemaira in subjects with emphysema due to alpha1-proteinase inhibitor deficiency.
clinicaltrials.gov/ct2/show/NCT00261833, last update posted 19-09-2015, accessed 25-06-2018
under
EXACTLE
Dirksen A, Piitulainen E, Parr D, et al. 2009. Exploring the role of CT densitometry: a randomised s
Care.tudy of
(CTH)
augmentation therapy in alpha1-antitrypsin deficiency,
Eur Respir J, 33(6): 1345-53.
Parr DG, Dirksen A, Pi tulainen R, et al. 2009. Exploring the optimum approach to the use of CT
1982 Aged
densitometry in a randomised placebo-controlled study of augmentation therapy in alpha 1-antritypsin
deficiency,
Respir Res, 10: 75.
released
Act and
NCT00263887, Alpha-1-Antitrypsin (AAT) To Treat Emphysema In AAT-Deficient Patients (EXACTLE).
clinicaltrials.gov/ct2/show/NCT00263887, last updated 21-08-2014, accessed 25-06-2018
been
DIRKSEN99
Dirksen A, Dijkman J, Madsen F, et al. 1999. A randomized clinical trial of alpha(1)-antitrypsin
augmentation therapy,
Am J Respir Crit Care Med, 160(5): 1468-72.
has
Health
Non-RCTs
of
(Karl et al.
Karl F, Holle R, Bals R, et al. 2017 Costs and health-related quality of life in Alpha-1-Antitrypsin Deficient
Information
2017)
COPD patients.
Respir Res, 18(1): 60.
of
(Barros-Tizon
Barros-Tizon, J C, Torres, M L, Blanco I, et al. 2012. Reduction of severe exacerbations and
et al. 2012)
hospitalization-derived costs in alpha-1-antitrypsin-deficient patients treated with alpha-1-antitrypsin
augmentation therapy,
Ther Adv Respir Dis, 6(2): 67-78.
document
(Tonelli et al.
Tonelli, A R, Rouhani F, Li N, et al. 2009. Alpha-1-antitrypsin augmentation therapy in deficient individuals
2009)
enrolled in the Alpha-1 Foundation DNA and Tissue Bank,
Int J Chron Obstruct Pulmon Dis, 4: 443-452.
This
Department
(Wencker et
Wencker, M, Fuhrmann, B, Banik, N, et al. 2001. Longitudinal follow-up of patients with alpha(1)-protease
Freedom
al. 1998)
inhibitor deficiency before and during therapy with IV alpha(1)-protease inhibitor,
Chest, 119(3): 737-744.
the
(Lieberman
Lieberman, J. 2000. Augmentation therapy reduces frequency of lung infections in antitrypsin deficiency:
2000)
a new hypot
by hesis with supporting data,
Chest, 118(5): 1480-1485.
(The Alpha-1-
The Alpha-1-Antitrypsin Deficiency Registry Study Group. 1998. Survival and FEV1 decline in individuals
Antitrypsin
with severe deficiency of alpha1-antitrypsin. The Alpha-1-Antitrypsin Deficiency Registry Study Group, Am
Deficiency
J
Respir Crit Care Med, 158(1): 49-59.
Registry Study
Group 1998)
(Seersholm et Seersholm N, Wencker M, Banik N, et al.1997. Does alpha1-antitrypsin augmentation therapy slow the
al. 1997)
annual decline in FEV1 in patients with severe hereditary alpha1-antitrypsin deficiency?
Wissenschaftliche Arbeitsgemeinschaft zur Therapie von Lungenerkrankungen (WATL) alpha1-AT study
group,
Eur Respir J, 10(10): 2260-2263.
Safety
The Alpha-1-
The Alpha-1-Antitrypsin Deficiency Registry Study Group. 1998. Survival and FEV1 decline in individuals
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
25
Page 39 of 218
FOI 5155 - Document 4
Trial
Reports
Antitrypsin
with severe deficiency of alpha1-antitrypsin. The Alpha-1-Antitrypsin Deficiency Registry Study Group,
Am
Deficiency
J Respir Crit Care Med, 158(1), pp. 49-59.
Registry Study
Group 1998
Barker et al.
Barker, AF, Siemsen, F, Pasley, D et al. 1994. Replacement therapy for hereditary alpha1-antitrypsin
1994
deficiency. A program for long-term administration,
Chest, 105(5), pp. 1406-1410.
Barker et al.
Barker, AF, Iwata-Morgan, I, Oveson et al. 1997. Pharmacokinetic study of alpha 1-antitrypsin infusion in
1997
alpha 1-antitrypsin deficiency,
Chest, 112(3), pp. 607-613.
Barros-Tizón
Barros-Tizon, JC, Torres, ML, Blanco, I et al. 2012. Reduction of severe exacerbations and
et al.
hospitalization-derived costs in alpha-1-antitrypsin-deficient patients treated with alpha-1-antitrypsin
2012
augmentation therapy,
Ther Adv Respir Dis, 6(2), pp. 67-78.
Campos et al. Campos, MA, Kueppers, F, Stocks et al. 2013. Safety and pharmacokinetics of 120 mg/kg versus 60
2013
mg/kg weekly intravenous infusions of alpha-1 proteinase inhibitor in alpha-1 antitrypsin deficiency: a
the
multicenter, randomized, double-blind, crossover study (SPARK),
COPD, 10(6), pp. 687-695.
Hubbard &
Hubbard, RC & Crystal, RG, 1988. Alpha-1-antitrypsin augmentation therapy for alpha-1-antitrypsin
Crystal1988
deficiency,
Am J Med, 84(6), pp. 52-62.
McElvaney et
McElvaney, NG, Burdon, J, Holmes, M et al. 2017. Long-term efficacy and safet
under y of alpha1 proteinase
al. 2017
inhibitor treatment for emphysema caused by severe alpha1 antitrypsin deficiency: an open
(CTH) -label
Care.
extension trial (RAPID-OLE),
Lancet Respir Med, 5(1), pp. 51-60.
Sandhaus et
Sandhaus, RA, Stocks, J, Rouhani et al. 2014, Biochemical efficacy and safety of a new, ready-to-use,
al. 2014
liquid alpha-1-proteinase inhibitor, GLASSIA (alpha1-proteinase inhibitor (human), intravenous), COPD,
1982 Aged
11(1), pp. 17-25.
released
Schmidt et al.
Schmidt, EW, Rasche, B, Ulmer et al. 1988. Replacement therapy for alpha-1-protease inhibitor
Act and
1988
deficiency in PiZ subjects with chronic obstructive lung disease,
Am J Med, 84(6), pp. 63-69.
been
Schwaiblmair
Schwaiblmair, M, Vogelmeier, C & Fruhmann, G, 1997. Long-term augmentation therapy in twenty
et al. 1997
patients with severe alpha-1-antitrypsin deficiency--three-year follow-up,
Respiration, 64(1), pp. 10-15.
has
Health
Stocks et al.
Stocks, J, Brantly, M, Wang-Smith, L et al. 2010. Pharmacokinetic comparability of Prolastin®-C to
of
2010
Prolastin® in alpha1-antitrypsin deficiency: a randomized study,
BMC Clin Pharmacol, 10pp. 13.
Information
of
Stoller et al.
Stol er, JK, Fal at, R, Schluchter, MD et al. 2003. Augmentation therapy with alpha1-antitrypsin: patterns
2003
of use and adverse events,
Chest, 123(5), pp. 1425-1434.
document
Wencker et al. Wencker, M, Banik, N, Buhl, R et al. 1998. Long-term treatment of alpha1-antitrypsin deficiency-related
1998
pulmonary emphysema with human alpha1-antitrypsin. Wissenschaftliche Arbeitsgemeinschaft zur
Department
Therapie von Lungenerkrankungen (WATL)-alpha1-AT-study group,
European Respiratory Journal, 11(2),
This Freedom
pp. 428-433. the
Wewers et al.
Wewers, MD, Casolaro, MA, Sellers, SE et al. 1987. Replacement therapy for alpha 1-antitrypsin
1987
deficiency associated with emphysema,
N Engl J Med, 316(17), pp. 1055-1062.
by
Information on current clinical trials was searched from Clinicaltrials.gov, Cochrane Central Register
of Controlled Trials, EU Clinical Trials Registry, WHO International Clinical Trials Registry Platform,
Current Controlled Trials MetaRegister, and the Australian New Zealand Clinical Trials Registry.
Search strategies for the clinical trial search are reported in Appendix B, and details of identified
trials are presented in Tables 10, 11 & 12.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
26
Page 40 of 218
FOI 5155 - Document 4
Overall, 11 clinical trials were identified as recruiting or active studies on A1PI augmentation therapy
(Tables 10, 11 & 12). Six of these were multinational trials. The largest estimated enrolment was 400
patients.
One A1PI clinical trial on the lung was marked as terminated because the intervention drug, Aralast,
was phased out of the market (NCT00313144). Otherwise, there seems to be no indication of
potential publication bias.
Table 10
Details of clinical trials identified on Clinicaltrials.gov
Trial ID;
Status
Design
Patient
Intervention Comparator
Outcome measure(s)
location
Estimated
Target N
indication
completion
the
NCT02525861 Recruiting
RCT
Alpha1-
GLASSIA:
GLASSIA:
Proportion AEs,
Canada,
May 2020
36
Proteinase
60mg/kg BW
60mg/kg BW
incidence treatment-
United States
Inhibitor
administered
administered
emergent ARs,
deficiency
at a rate of 0.2 at a rate of 0.2 proportion discontinued
under
mL/kg/min;
mL/kg/min;
treatments, propor
Care. tion
(CTH)
At lower
At higher
experiencing binding or
particulate
particulate
neutralising A1PI,
level
level
antigenic A1PIA levels,
1982 Aged
functional A1PI
released
NCT02722304 Recruiting
RCT
Alpha-1
ARALAST: 60 Placebo
Rate of change in lung
Act and
Australia,
July 2021
138
Proteinase
or 120 mg/kg
density
Canada,
Inhibitor < 8 body
been
United States
µM
weight/week
GLASSIA: 60
or 120 mg/kg
has
Health
weight/week
of
NCT02614872 Active
RCT
Planning to
GLASSIA
SOC
Incidence of AEs,
Information
Israel
December
30
undergo
treatment in
incidence /rate of acute
2020
l
of ung
addition to
rejection
transplant
SOC
NCT01983241 Recruiting
RCT
document Alpha-1 PROLASTIN- Placebo Change in baseline in
(SPARTA)
August 2021 339
Proteinase
C: 60 or 120
whole lung PD15
13 countries
Inhibitor <
mg/kg body
measured by CT scan
This
Department
11 µM
weight/week
Freedom
NCT02796937 Recruiting
Ope
the n-label Completed PROLASTIN- NA
Number AEs, number
(SPARTA-
by invitation extension
the
C: 60mg/kg
SAEs, discontinuation of
OLE)
July 2018
by of SPARTA SPARTA body
study due to AEs
7 countries
RCT
trial
weight/week
250
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
27
Page 41 of 218
FOI 5155 - Document 4
Trial ID;
Status
Design
Patient
Intervention Comparator
Outcome measure(s)
location
Estimated
Target N
indication
completion
NCT01974830 Recruiting
Prospective Requiring
NR
NA
Change in pulmonary
(AL1TER)
October
cohort
A1PI
function, including
United States 2020
study
therapy and
FEV1, at 1 year
400
agree to use
Coram's
home
infusion
services
Abbreviations:
A1PI = alpha-1 proteinase inhibitor,
AE = adverse events,
AR = adverse reactions,
NA = not applicable,
NR = not
reported,
PD15 = 15th percentile point,
RCT = randomised controlled trial,
SAE = serious adverse events,
SOC = standard of care.
Table 11
Details of clinical trials identified on EU Clinical Trials Registry
the
EudraCT
Design
Status
Start
Patient
Intervention
Comparator
Number
Estimated
date
indication
Location
enrolment
under
(CTH) Care.
2005-002402-36 Multi-centre,
Ongoing April
Alpha-1
A1PI
NA
Spain
open-label trial
2006
Antitrypsin
35
deficiency
Aged
released 1982
2007-004869-18 Case series
Ongoing July
Alpha-1
Prolastin
NA
Act and
Israel
26
2008
proteinase
inhibitor
been deficiency
has
Health
Table 12
Details of clinical trials identified on WHO International Clinical Trials Registry Platform
Information
of
Study ID
Design
Status
Start
Patient
Intervention
Comparator
Location
Estimated
date
indication
of
enrolment
EUCTR2015-
Multi-centre,
Authorised January
Patients
PROLASTIN-C
NA
document
004110-23-DK
open-label trial
2016
who have
16 countries
250
completed
Department participation
This Freedom
in SPARTA
the
Study
EUCTR2008-
RCT
Authorised August
Alpha-1
A1PI
Placebo
by
005326-36-GB 200
2009
antitrypsin
7 countries
deficiency
Abbreviations:
A1PI = alpha-1 proteinase inhibitor,
EUCTR = EU Clinical Trials Registry,
NA = not applicable.
APPRAISAL OF THE EVIDENCE
Appraisal of the evidence was conducted in four stages:
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
28
Page 42 of 218
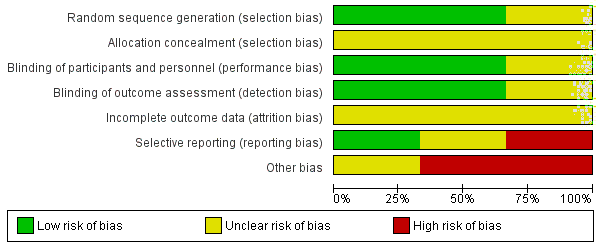
FOI 5155 - Document 4
Stage 1: Appraisal of the risk of bias within individual studies (or systematic reviews) included in the
review. Some risk-of-bias items were assessed for the study as a whole, while others were assessed
at the outcome level (Section B.3).
Stage 2: Extraction of the pre-specified outcomes for this assessment, synthesising (meta-analysing
or a narrative synthesis) to determine an estimate of effect per outcome.
Stage 3: Rating the overall quality of the evidence per outcome, across studies, based on the study
limitations (risk of bias), imprecision, inconsistency of results, indirectness of evidence, and the
likelihood of publication bias. This was done to provide an indication of the confidence in the
estimate of effect in the context of Australian clinical practice (Evidence profile tables, Table 102 and
Table 104 are presented in Appendix C).
the
Stage 4: Integration of this evidence to draw conclusions about the net clinical benefit of the
intervention in the context of Australian clinical practice (Sections B.6-8).
under
(CTH) Care.
B.3.
RISK OF BIAS ASSESSMENT
1982 Aged
RANDOMISED CONTROLLED TRIALS
released
The overall risk of bias in the core RCTs was low to moderate. S
Act ummary
and scores for the individual
domains of bias are presented in Figure 5 and Figure 6. The oldest trial was difficult to score due to
been
inadequate reporting of the study design (Dirksen et al. 1999).
has
Health
Information
of
of
document
This Freedom
Department
the
by
Figure 5
Summary of the overall risk of bias across the included studies
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
29
Page 43 of 218
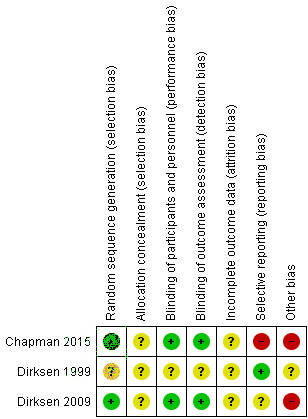
FOI 5155 - Document 4
the
under
(CTH) Care.
Aged
released 1982
Act and
Figure 6
Risk of bias in the included randomised controlled trials
been
Randomisation and al ocation concealment
has
Health
Both the RAPID and EXACTLE trials used an adequate method of randomising patients, conducted by
of
an independent third party, and there was no evidence of i
Information mportant baseline imbalances (Chapman
et al. 2015; Dirksen et al. 2009). The fin
of al trial stratified patients by age, level of FEV1 and nationality,
and randomised by the minimisation method (Dirksen et al. 1999). This was appropriate given the
relatively small sample size for the t
document rial (n=58). There were no obvious imbalances in the baseline
lung function or CT density, however, demographic characteristics (e.g. age, nationality, etc.) were
Department
not reported acros
This s treatment groups (Dirksen et al. 1999).
Freedom
the
The RAPID trial provided masked, sequential enrolment numbers to each site (Chapman et al. 2015).
by
Patients were assigned a consecutive number after meeting the requirements for study entry,
however, it is unclear how the treatments were masked to the study investigator responsible for
patient enrolment. In the EXACTLE trial, it was not reported who enrol ed patients into the study
(Dirksen et al. 2009). The pharmacists responsible for preparing medication were not blinded to the
treatment allocation. The final study did not report the method of allocation concealment (Dirksen
et al. 1999).
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
30
Page 44 of 218
FOI 5155 - Document 4
Blinding
Patients and investigators, including outcome assessors, were effectively blinded to the treatment
allocation in the RAPID and EXACTLE trials (Chapman et al. 2015; Dirksen et al. 2009). In both
studies, medication was prepared in opaque sleeves to the same volume per kg body weight in the
placebo and A1PI groups. Only the pharmacists preparing the medication were aware, or could
potentially have been aware, of the treatment allocation. The pharmacists had no interaction with
the patients or investigators. DIRKSEN99 was reportedly double-blinded, however, the method for
ensuring blinding was established and maintained was not reported (Dirksen et al. 1999).
Incomplete outcome data
Patient flow through the clinical trials is presented in Table 13.
the
The RAPID trial reported missing data in 10 A1PI patients (9.7%) compared to 20 placebo patients
(23.0%) (Chapman et al. 2015). The reasons for withdrawals were similar, but the total numbers
under Care.
were large enough to potentially have impacted the results. A modified intention-to
(CTH) -treat (mITT)
was reported, but it was unclear how missing data was accounted for in the analysis. The outcome
tables included the total number of patients randomised to each treatment arm, but not the number
1982 Aged
of patients included in the analysis.
released
Act and
There were 8% (3/38) versus 18% (7/39) losses to fol ow-up between A1PI and placebo groups in the
EXACTLE trial (Dirksen et al. 2009), although overall nu
been mbers were small.
Health
DIRKSEN99 reported two study drop-outs, fo
has r patients who resumed smoking (Dirksen et al. 2009). It
was unclear to which treatment arm these patients were
of assigned.
Information
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
31
Page 45 of 218
FOI 5155 - Document 4
Table 13
Patient flow in randomised controlled trials
Study ID
Length of study
Randomised,
Death,
Undergoing lung Discontinued due to Loss of follow-up
Analysed,
Intervention
(main paper)
n
n (%)
transplantation,
various reasons, reasons unspecified,
n (%) *
Comparator
n (%)
n (%)
n (%)
RAPID
the
α1-antitrypsin
24 months
93
1 (1%)
1 (1%)
6 (6%)
1 (1%)
84 (90%)
Placebo
24 months
87
3 (3%)
1 (1%)
14 (16%)
0 (0%)
69 (79%)
RAPID-OLE (non-
under Care.
randomised)
(CTH)
α1-antitrypsin
48 months
84
0 (0%)
0 (0%)
11 (13%)
23 (27%)
50 (60%)
Placebo
48 months
69
0 (0%)
0 (0%)
8 (12%)
14 (20%)
47 (68%)
1982 Aged
EXACTLE
released
α1-antitrypsin
24 months
38
0 (0%)
1 (3%)
1 (3%)
0 (0%)
36 (95%)
Act and
Placebo
24 months
39
0 (0%)
1 (3%)
5 (13%)
0 (0%)
33 (85%)
α1-antitrypsin
30 months
36
0 (0%)
1 (3%)
17 (47%)
0 (0%)
19 (53%)
been
Placebo
30 months
35
0 (0%)
2 (6%)
16 (46%)
0 (0%)
17 (49%)
DIRKSEN99
has
Health
α1-antitrypsin
36 months
28±2
0 (0%)
0 (0%)
±2 (7%)
0 (0%)
28 (100%)
of
Placebo
36 months
28±2
0 (0%)
0 (0%)
±2 (7%)
0 (0%)
28 (100%)
Information
*The RAPID trial reportedly conducted an ITT analysis for all of the included outcomes, however, it was unclear how these losses to follow-up were accounted for in the analysis.
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
32
Page 46 of 218
FOI 5155 - Document 4
Selective reporting
Several secondary outcome measures reportedly collected in the RAPID trial, based on the
clinicaltrials.gov entry, were not reported in the trial publication (Chapman et al. 2015). These
included per cent change in FEV1, per cent change in FEV1 as a ratio of FVC, change in lung density,
and severity of exacerbations (Chapman et al. 2015). The EXACTLE trial reported FEV1, DLCO and
diffusing coefficient for carbon monoxide (KCO) online only, noting that no significant differences
were observed. Mortality was measured but not reported (Dirksen et al. 2009). There were no
reporting issues with the final trial (Dirksen et al. 1999).
Other bias
the
Two of the trials had important conflicts of interest. The RAPID trial was deemed to have a high risk
of bias due to conflicts of interest (Chapman et al. 2015). The trial was funded by CSL Behring, the
manufacturer of Zemaira, and employees of the funding body were involved in the data analysis,
under Care.
data interpretation and writing of the trial publication. In addition, four of the study
(CTH) authors had
received consulting, research and/or personal funding from CSL Behring and other manufacturers of
A1PI products (i.e. Grifols). Funding for the EXACTLE trial was provided by the manufacturer of the
1982 Aged
study medication, Prolastin® (Talecris Biotherapeutics, Inc., Research Triangle Park, NC, USA)(Dirksen
released
et al. 2009). Two authors were employees of Talecris Bioth
Act erapeutics, Inc., however, their
and
involvement in the study is unclear. Editorial assistance was provided by an international
been
biopharmaceutical consulting organisation (PAREXEL, Worthing, UK), whose involvement was also
funded by Talecris Biotherapeutics, Inc. No information was provided about funding or conflicts of
has
Health
interest in the final trial (Dirksen et al. 1999).
Information
of
NON-RANDOMISED COMPARATIVE STUDIES
of
Seven non-randomised comparative studies were used in the assessment of safety and efficacy.
Three studies were interrupted time
document -series which compared lung function or infection pre and post
A1PI in a single population. Three studies used registry data or hospital medical records to
Department
retrospectively com
This pare patients treated with or without A1PI. One study evaluated the healthcare
Freedom
costs and health-related quality of life in COPD patients with and without AATD; a subgroup analysis
the
further classified COPD patients with AATD into those treated with or without A1PI.
by
The quality of the non-randomised comparative studies was appraised using the Risk of bias in non-
randomised studies of interventions (ROBINS-I) (Sterne et al. 2016b). According to this appraisal, all
seven studies were considered of serious risk of bias. All studies were graded serious in the “bias due
to confounding” section as they failed to report pertinent patient demographic details. Further,
most studies failed to accurately report the intervention and the follow-up time often differed
between groups. Most studies were of moderate bias in terms of the outcomes reported owing to
their retrospective design (the assessors where aware of the intervention each patient received).
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
33
Page 47 of 218
FOI 5155 - Document 4
Full results of the risk of bias appraisal are presented in Appendix D. A summary of the overall risk of
bias per item is presented in Figure 7.
Most studies retrospectively analysed patient data from either registries or patient records from
multiple sites. Reported characteristics of patients included in the studies were age, gender and
smoking status. The most common issue that may lead to bias was the limited patient demographic
information provided in the studies, with most omitting details regarding health status,
environmental exposure (toxin exposure or packet years), socioeconomic status and lung function.
Further, owing to the retrospective nature of trial data, there may be differences in the duration of
exposure between participants or change in practice to manage AATD. Studies attempted to limit
the influence of confounding variables on outcome measures by using multivariate random or fixed
the
effects modelling. However, this was not performed for all outcomes of interest nor did it include all
variables and consequently, some of the results were stil prone to bias. The number and severity of
adverse events were reported in one study (Barros-Tizon et al. 2012). The remaining studies
under
neglected this variable.
(CTH) Care.
Bias in selection of the reported results
1982 Aged
Bias in measurement of outcomes
released
Act and
Bias due to missing data
Bias due to departures from intended interventions been
Bias in measurement of intervention
has
Health
Bias selection of participants into the study
of
Bias due to confounding
Information
Overall B
of ias
0%
20%
40%
60%
80%
100%
document
Low risk of bias
Moderate risk of bias
Serious risk of bias
Department
Figure 7
Summ
This
ary of risk of bias across the included non-randomised studies
Freedom
the
OBSERVATIONAL TRIALS (SAFETY OUTCOMES)
by
Thirteen single-arm studies were used in the assessment of safety. Four of the single-arm studies
had a comparator group also receiving A1PI, the difference being dose or duration of AT. As the
intervention was not significantly different, they were treated as single-arm studies.
The quality of the single-arm studies was appraised using the IHE Quality Appraisal of Case Series
Studies (Guo et al. 2016). The open-label extension is referred to as a non-randomised study and its
quality was appraised using ROBINS-I (Risk Of Bias In Non-randomised Studies - of Interventions
(Sterne et al. 2016a)).
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
34
Page 48 of 218
FOI 5155 - Document 4
The 13 single-arm studies were appraised under the IHE Quality Appraisal tool. According to this
appraisal, eight studies were considered to have a high risk of bias, and five were considered to have
a moderate risk of bias. No studies were considered to have low risk of bias. Full results of the risk of
bias appraisal are presented in Appendix C. A summary of the overall risk of bias per item is
presented in Figure 8.
The studies were mostly prospective, multi-centre studies. Common issues that may lead to bias
include patients not being included consecutively, and wide variations in FEV standard deviation,
indicating that patients were not included at a similar point in the disease. Reported characteristics
of patients in the studies included age, gender, A1P1 serum concentration and smoking status. Most
studies did not report patient height weight and ethnicity data. Outcomes mostly were not assessed
using appropriate methods. It was expected that a method for reporting and grading th
the e severity of
adverse events would be described, but only two studies used such a method and two further
studies partially did so. Many studies (all but two) neglected to report estimates of random
under
variability in the data, although this is uncommon in adverse event reporting. The conclusions were
(CTH) Care.
supported by the results in all studies. Reporting of competing interests and sources of support were
evident in three studies only. The IHE Quality Appraisal of Case Series Studies is reported in Table
1982 Aged
107.
released
Act
Studies were considered to be at low risk of bias if 12-17 yes responses
and were given during the
appraisal, moderate risk of bias if 6-11 yes responses were recorded, and high risk of bias if 0-5 yes
been
responses were recorded. Inadequate length of fol ow-up (<12 months) was also used as an
automatic trigger for high risk of bias for the safety outcomes.
has
Health
of
The quality of the RAPID-OLE trial was appraised using the ROBINS-I appraisal tool. Low risk of bias
Information
was identified in relation to confounding, selection of participants into the study, adherence to
of
intended interventions, and missing data. Risk of bias in measurement of outcomes was considered
to be moderate, as the assessors were not blinded to the intervention and the outcome of al -cause
document
mortality could be subject to negligible assessor judgement. There was considered to be moderate
Department
bias in selection of reporting results, as while was there is no published protocol the outcomes are
This Freedom
consistent with an a priori plan, there is no indication of selection of reported patients of analyses.
the
The ROBINS-I appraisal is reported in Table 108.
by
Another potential for bias was identified in a systematic review retrieved in the scoping stage
(Sandhaus et al. 2016). The systematic review enquired if medical management of COPD should be
altered in patients with COPD due to AATD. The review found no reliable data suggesting a different
treatment response to BSC in COPD patients with or without AATD.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
35
Page 49 of 218
FOI 5155 - Document 4
17. Competing interests & sources of support
16. Conclusions supported by results
15. Adverse events reported
14. Estimated random variability in data analysis
13. Loss to fol ow up reported
12. Fol ow up sufficient
11. Outcomes measured appropriately
10. Outcome assessors blinded
9. Outcome measures established a priori
Criteria
8. Intervention clearly described
7. Similar point of entry in study
6. Inclusion/exclusion criteria
the
5. Characteristics described
4. Consecutive reqruitment
3. Multicentre study
under
2. Study conducted prospectively
(CTH) Care.
1. Hypothesis/aim/objective
0%
20%
40%
60%
80%
100%
Number of sing
1982
le arm stu
Aged
dies
Yes
No
Unclear
Partial
released
Act and
Figure 8
Summary of risk of bias across the included single-arm studies
been
B.4.
CHARACTERISTICS OF THE EVIDENCE BASE
has
Health
Full details on the individual studies included in the evidence base appear in Appendix C, Table 102.
of
Summaries are provided in Table 14 and Information
Table 15. Overall, the included RCTs had
of good applicability to the proposed population, except for
ethnicity. All patients in the RAPID and EXACTLE trials were Caucasian. DIRKSEN99 did not report the
document
ethnicity of trial participants, but patients were recruited from similar centres to the EXACTLE trial
(i.e. Danish A1 registry). Patient characteristics across the included RCTs were largely homogenous.
Department
The included patien
This ts were ex- or n
Freedom ever-smokers, with severe A1 deficiency (serum A1 ≤ 11 µM), and
emphysema. Mean baseline FE
the V1% predicted values ranged from 46% ± 20% to 50% ± 3%. Dosing
regimens differed across the included trials, from 60mg/kg per week (Chapman et al. 2015; Dirksen
by
et al. 2009) to 250mg/kg per month (Dirksen et al. 1999).
Table 14
Key features of the included RCTs comparing A1PI augmentation therapy with placebo
Trial/Study
N
Design/
Risk of Patient population
Key outcome(s)
Result used
duration
bias
in economic
model
• Serum A1 ≤11µM • Mortality
MC, R, DB
• FEV1 35% to 70% •
RAPID
180
QoL
24 mths
Low
predicted (mean
Yes
74% ± 12%)
• FEV1 % predicted
• Ex- or never-
• CT lung density
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
36
Page 50 of 218
FOI 5155 - Document 4
smokers
• Severe adverse events
• Death due to adverse
event
• Discontinuation due to
adverse events
• Serum A1 <11µM • QoL
• FEV1 ≥25% to
• FEV1 % predicted
MC, R, DB
≤80% predicted
EXACTLE
77
• CT lung density
24/30 mths
Low
(mean 47% ±
Not used
20%)
• Severe adverse events
• Ex- or never-
• Discontinuation due to
smokers
adverse event
• PiZZ phenotype
• FEV
•
1 30% to 80%
QoL
DIRKSEN99
58
MC, R, DB
the
36 mths
High
predicted (mean
• FEV1 % predicted
Not used
49% ± 20%)
• CT lung density
• Ex-smokers
Random effect model;
under
overall pooled
(CTH) Care.
presented;
Meta-analysis
315
heterogeneity analysis;
k=3
-
-
-
Not used
FEV1 % predicted, CT
lung density, D
1982 Aged
CO
analysed
released
Abbreviations:
CT = computed tomography,
DB = double blind;
FEV1 = forced expiratory volume in 1 second,
k = studies;
MC = multi-
Act
centre,
QoL= quality of life;
R = randomised.
and
The population demographics of the non-RCTs were fairly homogenous and generally represent the
been
proposed population for the intervention. Patients were mostly male (range, 50%–75%), ex-smokers
Health
(range, 78%–96%) with a PiZZ phenotype (
has range, 89%–100%). Patients predicted FEV1 % typically
of
ranged between 35%–40% however, the comparator group in Barros-Tizon et al. (2012) reported an
Information
average predicted FEV1 of 77%. Two and three studies included patients with other Pi* phenotypes
of
(0.8%–8%) and current smokers (5%–14%) respectively. Most studies did not list ethnicity, BMI or
serum A1 levels (however, eligibility criteria of serum A1 levels <11 or 12 µM was listed in two
document
studies). The most commonly reported augmentation therapy was Prolastin administered
intravenously at 60mg/kg (body weight) once per week. The comparator was no augmentation
This
Department
therapy, no further details were pro
Freedom vided regarding what the comparator constituted.
the
Table 15
Key features of the included studies assessing alpha-1 antitrypsin augmentation for safety outcomes
by
Trial/Study
N
Design/
Risk of Patient population
Key outcome(s)
Result used
duration
bias
in economic
model
Alpha-1-
•
Antitrypsin
Serum A1 <11µM • ΔFEV1
Deficiency
NR, OL, Coh
or PiZZ phenotype
927
• Survival
Not used
Registry Study
52 months Serious • Ex- or never-
Group 1998
smokers
• Serum A1 <11µM • Number of
Barros-Tizon
MC, NR, Coh
• PiZZ, PiSZ, other
exacerbations
et al. 2012
127
36 months Serious
Not used
phenotype
• Lung function
• Ex- or never-
• Adverse events
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
37
Page 51 of 218
FOI 5155 - Document 4
smokers, smokers • Costs associated with
hospitalisation
•
Karl et al.
MC, NR
Direct and indirect health
2017
2,186
care costs
1 year
Serious • Current, ex- and
never-smokers
Not used
• Health-related QoL
• PiZZ or PiSZ
Lieberman
phenotypes
• Number of infections
2000
143
NR
NA
Serious
Not used
• Ex- or never-
• Perceived benefit
smokers, smokers
• PiZZ phenotype
• Serum A1 ≤12µM
Seersholm et
• FEV1 37 to 42%
al. 1997
295
MC, NR, OL
3-5 years Serious
• ΔFEV1(mL/year)
Not used
predicted (mean)
• Ex- or never
the
smokers
• PiZZ phenotype
Tonelli et al.
• FEV1 43 to 77% • ΔFEV1(mL/year)
under
2009
164
R, NR, OL
42 months Serious
predicted (mean)
Not used
• Mortality (CTH) Care.
• Current or ex-
smoker
• PiZZ, PiSZ, other
Aged
phenotypes
1982
Wenker et al.
• Current, ex- and • Δ
released FEV1(mL/year)
2001
96
MC NR, Coh
98 months Serious
never-smokers
Not used
Act and
• FEV1 41%
predicted (mean)
been
Abbreviations:
AAT = alpha-1-antitrypsin,
Coh = cohort,
CS = case series,
DB = double-blind,
FEV1 = forced expiratory volume in 1
second,
k = studies;
MC = multi-centre,
NR = non-randomised,
OL = open label (unblinded),
R = randomised,
SB = single blind,
µM =
has
Health
micromolar.
of
*Including RCTs
Information
B.5.
OUTCOME MEASURES AN
of
D ANALYSIS
Additional details on the outcomes
document measured in the included studies appear in Appendix C. The
claimed benefit of A1PI is that it slows the progression of emphysema in patients that are A1PI
Department
deficient. Thus the primary outcomes of interest relate to monitoring the relative speed of disease
This Freedom
progression in patients treated with A1PI compared to those treated with BSC or placebo. The most
the
relevant clinical outcome for monitoring disease progression is the relative mortality rate over time,
by
however, mortality was investigated as a secondary outcome in the included studies, and they were
underpowered to detect significant differences in relative survival. In lieu of patient-important
outcomes, the RCTs were designed to detect changes in surrogate markers for disease progression.
PRIMARY EFFECTIVENESS OUTCOMES
The primary effectiveness outcomes defined in the included RCTs are outlined in Table 16. The
primary outcomes were independent, that is they were unaffected by clustering. The RAPID and
EXACTLE trials primarily aimed to measure disease progression using annual rates of CT-measured
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
38
Page 52 of 218
FOI 5155 - Document 4
lung density decline (Chapman et al. 2015; Dirksen et al. 2009), whereas the Dirksen et al. (1999)
trial investigated changes in FEV1 as the primary outcome. The primary effectiveness outcomes in
the non-RCTs are outlined in Table 16. The outcomes differed substantial y between the included
trials. ΔFEV1 and mortality/survival were the most frequently reported outcomes (four and two
studies respectively). One study each addressed healthcare costs, exacerbations and the number of
infections.
Table 16
Primary outcomes and statistical analyses of the randomised and non-randomised controlled trials
Trial ID
Definition of primary outcome
Method of primary statistical analysis
RCTs
RAPID
Annual rate of decrease in lung density,
Mixed-effects regression model, adjusted PD15 at
Chapman et al. calculated from the shift in the 15th percentile of baseline, 3, 12, 21 and 24 months.
the
(2015)
A
CT lung density (PD15), measured as a
combination of TLC and FRC.
EXACTLE
Progression rate of emphysema determined by Linear regression on time of PD15 measurement in a
Dirksen et al.
change in PD15, measured by annual CT scan random coefficient regression model.
under Care.
(2009)
B
of TLC.
(CTH)
DIRKSEN99
Annual mean changes in FEV1
Random-effects regression model with FEV1 and CT
Dirksen et al.
densitometry parameters as effect variables, and time,
(1999)
C
nationality and treatment group as explanatory variables.
1982 Aged
Non-RCTs
released
Act
Alpha-1-
Annual mean changes in FEV
and
1
FEV1: linear mixed effects modelling (covariates: mean
Antitrypsin
Survival
FEV1 % predicted)
Deficiency
Survival: kaplan-meier, log-rank test, cox proportional
been
Registry Study
hazards regression (covariates: baseline FEV1 % and
Group 1998
time)
has
Health
Number of exacerbation (worsening of the
Multivariate logistic regression
Barros-Tizon et patients basal condition which requires a of
al. 2012
change of the patient’s COPD medical
Information
regimen).
of
Generalised linear model (covariates: GOLD grade, age,
Karl et al. 2017 Direct and indirect health care costs
sex, education, smoking status, BMI, comorbidities)
document
Lieberman 2000 Number of infections per year
Chi squared test
This
Department
Seersholm et al. Annual mean changes in FEV1
Random effects modelling (covariates: age at baseline,
Freedom
1997
follow-up time, treatment, gender, initial FEV1, individual
the
patients)
Annual mean changes in FEV1
FEV1: random effects modelling (covariates: gender, age
by
Tonelli et al.
Mortality
at baseline, smoking status, individual patient, follow-up
duration)
2009
Mortality: logistic regression (covariates: age, gender,
baseline FEV1, COPD, smoking status)
Wenker et al. Annual mean changes in FEV1
Mixed effects modelling (covariates: treatment, individual
2001
patient)
Abbreviations:
COPD = chronic obstructive pulmonary disease;
CT = computed tomography;
FEV1 = forced expiratory volume in one
second;
FRC = functional residual capacity,
PD15 = 15th percentile density,
TLC = total lung capacity.
A The RAPID trial was powered to detect a dif erence in PD15 of 1.07 g/L per year across treatment groups, however, the clinical
importance of this dif erence has not been established in the literature.
B The EXACTLE trial was not powered to detect a dif erence in PD15 across treatment groups.
C The Dirksen et al. (1999) trial was powered to detect a mean change in FEV1 of at least 50% between groups at three years.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
39
Page 53 of 218
FOI 5155 - Document 4
The RAPID and EXACTLE trials measured lung density according to the change in the 15th percentile
lung density (PD15) value, adjusted using physiological volume correction. PD15 is a measurement of
lung density (measured in Hounsfield units or grams per litre), at which 15% of voxels, or pixels, on a
CT scan have lower densities (Parr et al. 2006). Volume correction was applied by calculating total
lung volume (measured on CT), divided by the individual patient’s predicted total lung capacity (TLC),
or maximal inhalation (Chapman et al. 2015; Dirksen et al. 2009). The RAPID trial also measured
density at functional residual capacity (FRC) or full expiration, as well as a combination measure
averaging TLC and FRC. DIRKSEN99 also recorded CT-measured lung density as a secondary outcome
(Dirksen et al. 1999).
PD15 has been validated as a consistent measure of lung density and marker of emphysema
the
progression (Parr et al. 2006). Research into CT-measured lung density decline has been conducted
specifically on A1PI deficient patients in order to overcome the challenges of adequately powering a
study to detect significant differences in functional outcomes such as FEV1 (Parr et al. 2006;
under
Schluchter et al. 2000); however, minimum clinically important differences (MCID) in
Care. CT
(CTH)
densitometry for monitoring disease progression are not yet defined in the literature.
C
1982 Aged
ORRELATION BETWEEN CT DENSITOMETRY, FUNCTIONAL AND PATIENT RELEVANT OUTCOMES
released
The published literature was searched to ascertain whether CT densitometry correlates with
Act and
functional outcomes, thereby providing indirect evidence of clinical improvement. PubMed was
searched on 23 July 2018 with the terms “Alpha 1
been anti-trypsin deficiency AND CT”. The search
identified 110 results, of which 12 studies reported on correlations between CT density and FEV1, KCO
has
Health
(lung function measures), mortality and quality of life (Table 17).
Information
of
There are, however, confounding variables that limit the generalisability of the results, for example
of
the different method of assessing lung density and the lung zones examined. A recent meta-analysis
assessing the relationship between CT densitometry and clinical outcomes in patients with COPD or
document
AATD, supports these findings (Crossley et al. 2018). It is worth noting that many of the studies listed
above form part of the evidence based used in this meta-analysis.
This Freedom
Department
The meta-analysis determined that FEV1 and KCO gas transfer correlated with CT density (Crossley et
the
al. 2018), although there was a high degree of heterogeneity among studies, attributable to the
by
different acquisition parameters.
Six studies reported on mortality (Crossley et al. 2018). The study concluded that it was
inappropriate to conduct a meta-analysis on this variable, even though each of the reported studies
found a correlation/association between densitometry and mortality when assessed individually.
Five studies addressed quality of life as scored by the St George Respiratory Questionnaire (Crossley
et al. 2018). The study authors concluded that it was inappropriate to conduct a meta-analysis on
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
40
Page 54 of 218
FOI 5155 - Document 4
this variable. Two studies found no relationship between CT density and quality of life, while three
studies demonstrated an association between the two variables.
Overall, the results suggest that CT density (either PD15 or -950 hounsfield units) generally
correlates with lung function measures (FEV1 and KCO) and mortality. However, the studies were
inconsistent regarding correlations between CT lung density and quality of life (Table 18). Stolk et al.
(2003) and Dowson et al. (2001) found a correlation between CT lung density and St George’s
Respiratory Questionnaire (SGRQ) whereas Dirksen et al. (2009) did not.
Table 17
Studies assessing correlation between CT lung density and function markers in AATD patients
Reference
Patient details %LAA
Statistical
Variable
Results
p-value
parameter
technique
adjusted for
the
FEV1 and KCO
Bernspang et al.
Swedish
PD15
Univariate and Diffusing
PD15 correlated FEV1
p < 0.05
(2011)
infants born
multivariate
capacity,
with FEV1
under
between 1972
regression
FEV
Care.
1 and
(CTH)
– 1974
analysis
PD15
(n=53)
Stolk et al. (2003) 10 AATD
PD15
Spearman
CT-derived
PD15 correlated Adjusted
Aged
registries in
correlation
lung volume wit
1982 h FEV1 and FEV1
p <
The
KCO when
0.0001
released
Netherlands,
adjusted by
KCO
p <
Act and
UK, Sweden,
lung volume.
0.0001
Canada,
Australia, New
been
PD15 correlated Not adjusted
Zealand,
with FEV1 (r =
FEV1
p <
Switzerland,
0.34) and
KCO
0.0001
has
Health
Spain,
(r = 0.29) not
KCO
p <
Belgium and
of
adjusted to
0.0001
Germany
TLC
Information
(n=226)
of
Dirksen et al.
AATD
PD15
Random
PD15 correlated FEV1
p =
(2009)
registries in
coefficient
with FEV1
0.007
Denmark,
model
PD15 not
document
Sweden and
correlated with
KCO
p = NR
UK
KCO
(n=77)
This
Department
Parr et al. (2006)
UK centre
PD
Freedom 15
Spearman
Upper zone
Upper zone
(n=74)
-950
correlation
PD15 and -950
PD15
p =
the
and the
index
0.001
Jonckheere-
correlated
-950
p =
by
Terpstra
FEV1
0.012
test
Lower zone
Lower zone
PD15
p = 0.35
PD15 and -950
-950
p = 0.09
index did not
correlate with
FEV1
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
41
Page 55 of 218
FOI 5155 - Document 4
Reference
Patient details %LAA
Statistical
Variable
Results
p-value
parameter
technique
adjusted for
Stolk et al. (2003) Dutch centre
PD15
Spearman
PD15 and -950
PD15
(n=22)
-950
correlation
correlated with FEV1
p =
FEV1 and
KCO
0.001
KCO
p = 0.007
-950
FEV1
p =
0.001
KCO
p = 0.004
Dowson et al.
UK centre
-910
Spearman’s
Upper and
For all
(2001)
(n=111)
rho test
lower zone
variables
p <
the
inspiratory and 0.001
expiratory CT
density
correlated with
under
FEV
Care.
1 and
KCO
(CTH)
Dirksen et al.
AATD
PD15
Pearson’s
PD15 correlated
KCO
p = 0.02
(1999)
registries in
correlation
with
KCO but
FEV1
p = 0.39
The
not
1982 FEV
Aged
1
Netherlands
released
and Denmark
Act
(n=56)
and
Survival
Green et al.
UK A1ADT
PD
been
15
Univariate and FEV1,
Lower but not
Lower zone
p
(2016)
registry
-910
multivariate
lower zone
upper zone CT = 0.042
(n=110)
regression
density
density
Upper zone
p
has
Health
analysis
decline,
associated with = 0.072
of whole lung mortality
density
Information decline
Dawkins et al.
ADAPT
-910
of Cox
Age
Upper zone CT Age corrected
(2009)
(n=488)
regression
density
analysis
analysis
associated with
p = 0.008
document
all-cause
mortality
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
42
Page 56 of 218
FOI 5155 - Document 4
Reference
Patient details %LAA
Statistical
Variable
Results
p-value
parameter
technique
adjusted for
Dawkins et al.
UK centre
-910
Univariate and Age, lower
Univariate
Univariate
(2003)
(n=256)
multivariate
and upper
Upper and
Upper ins
p =
regression
expiratory
lower zone CT
0.005
analysis
scan
density
Upper ex
p =
associated with 0.001
Odds ratio
all-cause
Lower ins
p =
mortality.
0.007
Lower ex
p =
Multivariate
0.002
Upper
expiratory
Multivariate
associated with Upper all-
all cause and
cause
p =
the
respiratory
0.001
mortality
Upper
respiratory
p
under
= 0.001
(CTH) Care.
Quality of life
1982 Aged
Vijayasaratha and ADAPT
PD15
Spearman
PD15 correlated Length in
released
Stockley (2012)
(n=23)
correlation
with
days
p =
Act
ex
and acerbation 0.003
length in days;
Delay of
delay of
antibiotics in
been
antibiotics in
days
p <
days; day 1
0.001
Health symptom
Day 1
has
scores but not
symptom
of
resolution
scores
p =
Information
length
0.035
Resolution
of
length
p = NR
Dirksen et al.
AATD
PD
document 15
Random
PD15 not
SGRQ
p = NR
(2009)
registries in
coefficient
correlated with
Denmark,
model
SGRQ
Department
Sweden and
This Freedom
UK
Stolk et al. (2003) Dutch centre
the PD15
Spearman
PD15 and -950
PD15 SGRQ
p
(n=77)
-950
correlation
correlated with = 0.028
by
SGRQ
-950 SGRQ
p
= 0.018
Dowson et al.
UK centre
-910
Spearman’s
CT density
SGRQ
p <
(2001)
(n=111)
rho test
correlated with 0.001
all domains of
SF-36
p <
SGRQ and SF- 0.05
36
Abbreviations:
AATD = alpha-1-antitrypsin deficiency,
CT = computed tomography,
EX = expiratory,
FEV1 = forced expiratory volume in
1 second,
Ins = inspiratory,
KCO = carbon monoxide transfer coefficient,
LAA% = low attenuation area %,
NR = not reported,
PD15 =
volume-adjusted 15th percentile density,
R = Pearson’s correlation coefficient,
SF-36 = 36 item short form survey,
SGRQ = St George
Respiratory Questionnaire,
TLC = total lung capacity.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
43
Page 57 of 218
FOI 5155 - Document 4
SECONDARY EFFECTIVENESS OUTCOMES
The secondary effectiveness outcomes measured in the direct randomised trials are outlined in
Table 18. Key outcomes are described below, noting that additional outcomes wil be defined for the
final report.
Table 18
Secondary outcomes and statistical analyses of the direct randomised trials
Trial ID
Definition of secondary outcomes
RAPID
1. FEV1
Chapman et al.
2. A1PI concentrations (functional and antigenic assays)
(2015)
3. Single-breath dif usion capacity (DLCO )
the
4. Incremental shuttle walk
5. Quality of life (SGRQ)
6. Mortality
under
7. Adverse events: Any untoward medical event occurring during the trial, defined as not relat
Care. ed,
(CTH)
possibly related, probably related, or related to A1PI augmentation.
EXACTLE
1. FEV1
Dirksen et al.
2. Dif using capacity of the lung for carbon monoxide (
D 1982 Aged
LCO)
(2009)
released
3. Transfer coefficient of the lung for carbon monoxide (
KCO)
Act and
4. Frequency of exacerbations collected in diary
5. Mortality
been
6. Quality of life (SGRQ)
Health
DIRKSEN99
7. Diffusion capacity (
D
has
LCO) at 3-month intervals
Dirksen et al.
8. Carbon monoxide dif usion constant (
K
of CO)
(1999)
Information
9.
Patient-administered serial spirometry daily
of
10. CT lung density
Abbreviations:
A1PI = alpha-1 proteinase inhibitor,
CT = computed tomography,
DLCO = dif using capacity for carbon monoxide,
FEV1 =
document
forced expiratory volume in one second,
KCO = carbon monoxide transfer coefficient,
SGRQ = St George’s Respiratory Questionnaire.
Details on the outcomes measured in the included
Department studies, along with the statistical methods used
This Freedom
to analyse the results, appear in Appendix D, Table 97 and Table 98.
the
QUALITY OF LIFE by
Quality of life using the St. George’s Respiratory Questionnaire (SGRQ) was reported in two of the
direct randomised trials. The SGRQ is a disease-specific questionnaire that measures QoL in patients
with obstructive airways disease. The questionnaire ranges from 0 to 100, with higher scores
indicating greater limitations and worsening quality of life. A mean change score of 4-8 units is
associated with a slightly efficacious treatment, 8-13 units for moderately efficacious treatment, and
13-16 units for very efficacious treatment (Jones 1994).
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
44
Page 58 of 218
FOI 5155 - Document 4
MORTALITY
The assessment of mortality separates patients into two groups; those which are dead and those
which are alive. The effects of an intervention on death can be calculated by examining the per cent
of patients alive or dead during follow-up, or model ing the rate of death using survival curves. Two
non-RCTs and one RCT reported the overall mortality rate following augmentation therapy
(Chapman et al. 2015; Tonelli et al. 2009; Alpha-1 registry study group 1998). One non-RCT used
survival curves (Kaplan-Meier) to determine in which patient’s augmentation therapy was most
effective (Alpha-1 registry study group 1998).
EXACERBATIONS/HOSPITALISATION DUE TO EXACERBATIONS
There are multiple definitions of exacerbations. For example, (Calverley 2005) defines
the exacerbations
as “an episode where a patient seeks medical help rather than a predefined change in one or more
symptoms”. However, exacerbations can be further stratified by frequency and severity and often
under
need to take into account baseline disease severity (Chapman et al. 2013). Given this, the MCIDs for
(CTH) Care.
exacerbations in COPD patients vary substantially with studies demonstrating a reduction in
exacerbations of 4 – 20% to be clinically meaningful (Chapman et al. 2013).
1982 Aged
Exacerbations were reported in three trials (two RCTs and one non-R
released CT). One RCT and one non-RCT
Act
used a similar definition of exacerbation: the worsening of a patient’s cond
and ition beyond normal day-
to-day variation which requires a change to their medical regimen (Dirksen et al. 2009, Barros-Tizon
been
et al. 2012). The RCT by Chapman et al. (2015) determined exacerbations in accordance with
(Anthonisen et al. 1987) who categorised exacerbations based
Health on the type and number of
has
symptoms.
Information
of
EXERCISE CAPACITY
of
Incremental Shuttle Walk
document
The incremental shuttle walk is an assessment of exercise capacity. The test requires the patients to
Department
walk up and down at 10m course. The speed at which a patient walks is dictated by an audio signal.
This Freedom
The lapse between each audio signal decreases every minute requiring the patient to walk
the
incremental y faster. The end of the tests occurs when the patient is too breathless to continue; they
by
fail to reach complete the course in the allocate time or they reach of 85% of their predicted heart
rate (Singh et al. 1992). The MCID for the incremental shuttle test in COPD patients is 48m (Singh et
al. 2008). One RCT used the incremental shuttle walk test to infer exercise capacity, however the
precise methodology was not reported (Chapman et al. 2015). The results of the study exhibited
considerably variability among the included population as indicated by the standard deviations.
DYSPNOEA
Dyspnoea as defined by the American Thoracic Society (1999) is the “subjective experience of
breathing discomfort that consists of qualitatively distinct sensations that vary in intensity”.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
45
Page 59 of 218
FOI 5155 - Document 4
Dyspnoea is typically a patient reported event, however, measurements of gas transfer, spirometry,
exercise ability and Borg or VAS scale can help diagnose dyspnoea. Dyspnoea was reported in one
RCT (Chapman et al.2015). However, the methodology used to assess dyspnoea was not reported.
LUNG FUNCTION
DLCO/KCO
The diffusing capacity of the lungs for carbon monoxide (DLCO or TLCO) and the carbon monoxide
transfer coefficient (KCO) are measures of lung function. They assess the transfer of inspired carbon
monoxide from the alveoli to the red blood cells in circulation. KCO additionally takes into account
the alveolar volume (the number of contributing alveolar units) and is calculated as DLCO/VA. The
the
MCID for DLCO as reported by (Horita et al. 2015) was 1.1 ml/min/mmHg (11% of baseline DLCO) when
anchored to FEV1 and six minute walk test. MCIDs for KCO were not found in our literature search. Al
three RCTs reported DLCO and two reported KCO. Dirksen et al. (1999 and 2009) assessed DLCO and KCO
under
in accordance with European guidelines (Quanjer et al. 1993). Chapman et al. (2015) d
(CTH) id not re
Care. port
KCO or the method used to measure DLCO. One non-RCT reported DLCO (Barros-Tizon et al. 2012), again
however, the methodology was not specified.
Aged
released 1982
FEV1
Act and
FEV1 is a functional outcome representing the amount of air forcibly expired within one second. It
been
was measured accurately in the included trials, using standard spirometry protocols. FEV1 has noted
limitations in disease-modification trials, however, because it changes slowly over time (therefore
has
Health
requiring long follow-up, generally > 2 years), it exhibits individual variability and until certain
of
thresholds are reached has limited correlation with endpoints such as mortality or exacerbations
Information
(Chorostowska-Wynimko 2016), and it requires large sample sizes to sufficiently power trials to
of
detect this effect (Schluchter et al. 2000). Literature defines 100 mL as the minimum clinically
important difference (Donohue 2005).
document
FEV1 was measured in four non-RCTs. Two studies
Department did not report the methodology use to assess
This Freedom
spirometry (Barros-Tizon et al. 2012, Wencker et al. 1998). The Alpha-1 registry study group (1998)
the
measured FEV1 before and after broncholdilator treatment and al owed subjects to perform up to
eight expirations to gen
by erate three reproducible scores. In Seersholm et al. (1997) FEV1 was
calculated in accordance with European respiratory society recommendations. However, the
intervention cohort measured FEV1 after two puffs of salbutamol and repeated FEV1 measurements
at least three times. It is unclear whether the control cohort underwent a similar methodology.
ADVERSE EVENTS
Adverse events (any, severe, and treatment-related), dyspnoea in particular, hospitalisation,
discontinuation, and death, were recorded as descriptive statistics. Most studies reported both the
number of events and the proportion of the patient population experiencing the event. Nine studies,
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
46
Page 60 of 218
FOI 5155 - Document 4
including the RCTs, recorded the events prospectively with outcome measures established a priori.
The remaining eight studies either recorded the events retrospectively (five studies) or only partially
established outcome measures before study commencement (three studies). It is unclear whether
important adverse events were captured in the retrospective trials; there was significant variation
between reported adverse event rates between prospective and retrospective studies. These are
highlighted in Section B.6.
META-ANALYSIS
Continuous outcomes were evaluated as mean differences or standardised mean differences.
Standardised mean differences were calculated in order to account for differences in measurement
scales across included studies. Dichotomous outcomes were evaluated using relative risks and
the
associated 95% confidence intervals.
Missing standard deviations were evaluated from available standard errors using the following
under
formula:
(CTH) Care.
SD = SE x √N
1982 Aged
Missing standard deviations were evaluated from available 95%
released confidence intervals using the
following formula:
Act and
been
SD = √N x (upper limit – lower limit) / 3.92
Health
Heterogeneity across studies was evaluated
has using I2 statistics. Fixed-effects models were used for
of
meta-analyses. Meta-analyses were conducted using RevMan version 5.3.
Information
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
47
Page 61 of 218
FOI 5155 - Document 4
B.6.
RESULTS OF THE SYSTEMATIC LITERATURE REVIEW
IS IT SAFE?
Summary – What is the safety status of A1PI augmentation therapy relative to best standard care?
Safety outcomes reported in the included studies were death due to adverse events, severe adverse events,
treatment-related adverse events, any adverse events, dyspnoea, infection from treatment, and
hospitalisation/discontinuation due to adverse events. There were no significant dif erences in the rates of these
outcomes identified in the direct RCTs.
Death was uncommon. Six deaths occurred in the eligible studies (899 patients), none of which were reported to
the
be treatment related.
Severe adverse events were uncommon, with a median occurrence of 2.1% in the patient population (range 0.0-
30.0%) across eleven studies. Discontinuation due to adverse events had a median occurrence of 0.6% in the
under Care.
patient population (range 0.0-7.1%) across nine studies; and hospitalisation due to adverse event
(CTH) s had a median
occurrence of 1.4% in the patient population (range 0.0-14.3%) across four studies.
Al studies found the same rates of severe adverse events across intervention groups. Overal , it appears that the
1982 Aged
intervention is safe, with most events being related to the underlying disease.
released
Act and
Seventeen studies were included for the evaluation of safety outcomes: the three RCTs also included
for effectiveness (Chapman et al. 2015; Dirksen et
been al. 1999; Dirksen et al. 2009), two open-label
extensions of RCTs (Campos et al. 2013; McElvaney et al. 2017), two further nRCTs with comparator
has
Health
groups still taking a form of A1PI (varied by dose, product or time frame) (Sandhaus et al. 2014;
of
Stocks et al. 2010a), and ten single-arms studies (Barker et al. 1997; Barker et al. 1994; Barros-Tizon
Information
et al. 2012; Hubbard and Crystal 1988; Schmidt et al. 1988; Schwaiblmair et al. 1997; Stoller et al.
of
2003; The Alpha-1-Antitrypsin Deficiency Registry Study Group 1998; Wencker et al. 1998; Wewers
et al. 1987).
document
Key safety outcomes reported in the included studies were death due to adverse events, severe
This
Department
adverse events, treatment-relat
Freedom ed adverse events, any adverse events, dyspnoea, and
hospitalisation or discontinua
the tion due to adverse events. Summaries of these outcomes across
studies are presented in Tables 17 to 23. Detailed safety outcomes in each study are presented in
by
Appendix D, Table 109 and Table 110. In addition, Table 26 shows severe adverse events across
studies on the particular intervention products, Zemaira and PROLASTIN-C. Because this evidence
was limited, studies eligible for the safety assessment included patients receiving AT with any A1PI
product. All considerations of safety and adverse effects should take this into account.
Meta-analysis was not conducted for the single arm studies due to differences in the duration of
follow-up and the populations included across studies. A narrative summary of the primary safety
outcomes is presented below.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
48
Page 62 of 218
FOI 5155 - Document 4
CONTRAINDICATIONS
AT with Prolastin or Zemaira is contraindicated in patients with a history of severe or anaphylactic
response to A1PI products, as well as any individuals with a known hypersensitivity to any of its
components (Aventis Bering 2003). All inclusion criteria reflected this. Patients with both AATD and
severe IgA deficiency are at risk of anaphylactic reaction and should not be treated (Alpha 1
Foundation 2015).
DEATH DUE TO ADVERSE EVENTS
Eight studies provided evidence on death due to adverse events. Six deaths occurred in the eligible
studies, which included 899 patients in total. In the single arm studies, death had a mean occurrence
of 2.3%, and a median occurrence of 0.0% in the patient population (range 0.0-7.1%). T
the he total rates
of death due to adverse events are presented in Table 19. For more detail on adverse events
reported in the studies see Appendix D, Tables 101 & 102.
under
(CTH) Care.
Table 19
Results of death due to adverse events across the included randomised controlled trials and single-
arm studies
RCT ID
Risk of bias Follow-up Treatment
Intervention
Comparator
Relative
1982 Aged
dose/frequency
rate (proportion) rate (proportion) difference
released
RR (95% CI)
Act and
RAPID
Low
24 months 60mg/kg per week 1/93 (1.0%)
3/87 (3.4%)
0.31 (0.03 to 2.94)
(Chapman et
al. 2015)
been
Single-arm Risk of bias Follow-up Treatment
Intervention
NA
NA
study ID
dose/frequency
rate (proportion)
has
Health
Barker et al. High
48 months 60mg/kg every 1-2 1/14 (7.1%)
NA
NA
of
1994
weeks Information
Barker et al. High
4 months
120mg/kg every 2 1/23 (4.3%)
NA
NA
of
1997
weeks
Barros-Tizón Moderate 18 months 60mg/kg every 1-3 0/127 (0.0%)
NA
NA
et al.
week
document s
2012
Campos et High
4 months
60mg/kg per week
Department 0/30 (0.0%) NA
NA
This
al. 2013
Freedom
Schwaiblmair High
36 months
the 60mg/kg per week 1/20 (5.0%) NA
NA
et al. 1997
by
Stocks et al. High
6 months
60mg/kg per week 0/24 (0.0%)
NA
NA
2010
Wencker et Moderate 6 years
60mg/kg per week 0/443 (0.0%)
NA
NA
al. 1998
Abbreviations:
CI = confidence interval,
NA = not applicable,
RCT = randomised controlled trial,
RR = relative risk.
The single RCT reported that death due to adverse events occurred in 2% of patients in the
intervention group and 3% in the placebo group. The four deaths were due to respiratory failure,
sepsis, pneumonia and breast cancer. Four single-arm studies reported that no patients died due to
adverse events (Barros-Tizon et al. 2012; Campos et al. 2013; Schwaiblmair et al. 1997; Stocks et al.
2010a). Two studies reported death of a patient due to adverse events after receiving Zemaira
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
49
Page 63 of 218
FOI 5155 - Document 4
(Barker et al. 1997; Barker et al. 1994). One of these resulted from progression of the disease (Barker
et al. 1994), and the other was noted to be unrelated to the intervention (Barker et al. 1997).
Overall, none of the deaths were reported to be treatment-related.
SEVERE ADVERSE EVENTS
Thirteen studies provided evidence on severe adverse events occurring after treatment with the
intervention (Barker et al. 1997; Barros-Tizon et al. 2012; Campos et al. 2013; Chapman et al. 2015;
Dirksen et al. 2009; McElvaney et al. 2017; Sandhaus et al. 2014; Schmidt et al. 1988; Schwaiblmair
et al. 1997; Stocks et al. 2010a; Stoller et al. 2003; Wencker et al. 1998; Wewers et al. 1987). In the
RCTs, severe adverse events occurred less frequently in the A1PI arm than placebo overall (RR=0.83,
95% CI 0.57 to 1.19), noting that this difference was not statistically significant. In the single arm
the
studies, severe adverse events had a median occurrence of 2.1% in the patient population (range
0.0-30.0%). The total rates of severe adverse events are presented in Table 20. For more detail on
adverse events reported in the studies see Appendix D, Tables 101 & 102. The forest plot indicating
under
(CTH) Care.
pooled rate of severe adverse events is presented in Figure 9.
Table 20
Results of severe adverse events across the included randomised control ed trials and single-arm
studies
Aged
released 1982
RCT ID
Risk of bias Follow-up Treatment
Intervention
Comparator
Relative
Act
dose/frequency
and
rate (proportion) rate (proportion) difference
RR (95% CI)
been
RAPID
Low
24 months 60mg/kg per week
28/93 (30.1%)*
28/87 (32.0%)*
0.94 (0.61 to
(Chapman et
1.44)
al. 2015)
has
Health
EXACTLE Low
30 months 60mg/kg per week
10/38 (26.3%)*
18/39 (46.1%)*
0.62
of
(Dirksen et
(0.31 to 1.23)
Information
al. 2009)
of
Non-RCT ID Risk of bias Follow-up Treatment
Intervention
NA
NA
dose/frequency
rate (proportion)
Barker et al. High
4 months
120mg/kg every 2
2/23 (8.7%)
NA
NA
document
1997
weeks
Barros-Tizón Moderate 18 months 60mg/kg every 1-3
4/127 (3.1%)†
NA
NA
Department
et al.
This
weeks
Freedom
2012
the
Campos et High
4 months
60mg/kg per week
0/30 (0.0%)
NA
NA
al. 2013
by
RAPID-OLE Moderate 48 months 60mg/kg per week
42/140 (30.0%)
NA
NA
(McElvaney
et al. 2017)
Sandhaus et High
3 months
60mg/kg (frequency 4/50 (8.0%)†
NA
NA
al. 2014
NR)
Schmidt et High
6 months
60mg/kg per week
0/20 (0.0%)
NA
NA
al. 1988
Schwaiblmair High
36 months 60mg/kg per week
0/20 (0.0%)
NA
NA
et al. 1997
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
50
Page 64 of 218
FOI 5155 - Document 4
Stocks et al. High
6 months
60mg/kg per week
2/24 (8.3%)
NA
NA
2010
Stoller et al. Moderate 7 years
Unclear
63/720 (events)
NA
NA
2003
Wencker et Moderate 6 years
60mg/kg per week
5/443 (1.1%)
NA
NA
al. 1998
Wewers et High
6 months
60mg/kg per week
0/21 (0.0%)
NA
NA
al. 1987
Abbreviations:
CI = confidence interval,
NA = not applicable,
NR = not reported,
RCT = randomised controlled trial,
RR = relative risk.
*Data obtained from results tab on the NIH’s clinical trials website. †Events reported to be “not related” to the intervention.
the
under
(CTH) Care.
Figure 9
Forest plot indicating the pooled rate of severe adverse events for A1PI compared to placebo
1982 Aged
Two RCTs, comprising a total of 257 patients, reported 84 severe adverse events in total. In the
released
RAPID trial, the placebo group (32.0%) experienced a slightly higher proportion of severe adverse
Act and
events than the intervention group (30.1%). Severe adverse events that occurred in more than 5% of
patients in this study population were chronic obs
been tructive pulmonary disorders, pneumonia and
lower respiratory infection.
has
Health
In the EXACTLE trial, the placebo group also experien
of ced a higher proportion of severe adverse
Information
events (46.1%) than the intervention group (26.3%). The trial reported that pneumonia, atrial
of
fibrillation, pulmonary embolism, and pneumothorax were severe adverse events that occurred in
more than 5% of patients in this study population.
document
The patients in McElvaney et al. (2017) who had received A1PI intervention for 24 or 48 months,
Department
reported here as a s
This ingle arm, experienced the same proportion of severe adverse events (30%). Five
Freedom
studies, with a total of 144 patients, reported that no severe adverse events had occurred. The
the
remaining single arm studies reported low rates of serioussevere adverse events (ranging from 1.1%
by
to 8.7%)
Notably, the EXACTLE, RAPID and RAPID/OLE trials reported higher rates of serious severe adverse
events than the single arm studies which were al had rates below ten percent. This may have been
due to the RCTs and NRCT being conducted prospectively, defining adverse events intentional y by
severity, and following patients more rigorously. Lengths of follow-up also might have played a part
as their follow-up points are longer than al but three of the eleven single arm studies.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
51
Page 65 of 218
FOI 5155 - Document 4
TREATMENT-RELATED ADVERSE EVENTS
Nine studies provided evidence on treatment-related adverse events (Barker et al. 1997; Barros-
Tizon et al. 2012; Campos et al. 2013; Chapman et al. 2015; Dirksen et al. 2009; McElvaney et al.
2017; Sandhaus et al. 2014; Schwaiblmair et al. 1997; Stocks et al. 2010a). The total rates of
treatment-related adverse events are presented in Table 21. For more detail on adverse events
reported in the studies see Appendix D, Tables 101 & 102.
In the RCTs, treatment-related adverse events occurred less frequently in the A1PI arm than placebo
overall (RR=0.86, 95% CI 0.57 to 1.29), noting that this difference was not statistically significant.
Treatment-related adverse events had a median occurrence of in the single arm studies of 10.0% in
the patient population (range 0.0-28.6%). Though, this relationship was drawn at the discretion of
the
the study authors and may not indicate true cause.
Table 21
Results of treatment-related adverse events across the included randomised controlled trials and
under
single-arm studies
(CTH) Care.
RCT ID
Risk of bias Follow-up Treatment
Intervention
Comparator
Relative difference
dose/frequency
rate (proportion) rate (proportion) RR (95% CI)
RAPID
Low
24 months 60mg/kg per week 21/93 (22.6%) 21/87 (24.1%) 0.
Aged 94 (0.55 to 1.59)
1982
(Chapman et
released
al. 2015)
Act and
EXACTLE Low
30 months 60mg/kg per week 11/38 (28.9%) 15/39 (38.5%) 0.75 (0.40 to 1.42)
(Dirksen et
al. 2009)
been
Single arm Risk of bias Follow-up Treatment
Intervention 1 Intervention 2 NA
study ID
dose/frequency
rate (proportion
Health
) rate (proportion)
has
Barker et al. High
4 months
120mg/kg every 2 4/23 (
of 17.4%)† NA
NA
1997
weeks
Information
Barros-Tizón Moderate
18 months 60mg/kg every 1-3 7/127 (5.5%)†
NA
NA
of
et al. 2012
weeks
Campos et High
4 months
60mg/kg per week 4/60 (6.7%)
NA
NA
al. 2013
document
RAPID-OLE Moderate
48 months 60mg/kg per week 18/180 (10.0%) NA
NA
(McElvaney This
Department
et al. 2017)
Freedom
Sandhaus et High
6 months
60mg/kg (frequency 6/21 (28.6%)†
NA
NA
the
al. 2014
NR)
by
Schwaiblmair High
36 months 60mg/kg per week 1/20 (5.0%)
NA
NA
et al. 1997
Stocks et al. High
6 months
60mg/kg per week 2/24 (8.3%)
NA
NA
2010
Abbreviations:
CI = confidence interval,
NA = not applicable,
NR = not reported,
RCT = randomised controlled trial,
RR = relative risk.
†unknown if patients were included for multiple events
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
52
Page 66 of 218
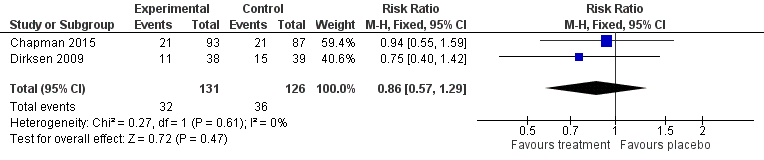
FOI 5155 - Document 4
Figure 10
Forest plot indicating rate of death due to adverse events in A1PI patients compared to placebo
The RAPID and EXACTLE trials reported rates of treatment-related adverse events that were similar
between intervention and placebo groups, and both slightly higher in the placebo group. The RAPID-
OLE trial (McElvaney et al. 2017), reported a rate of 10% for patients receiving Zemaira.
the
Treatment-related adverse events that occurred more than once in four studies (totalling 224
patients) included headache, dyspnoea and pruritus (Barker et al. 1997; Barros-Tizon et al. 2012;
under
Sandhaus et al. 2014; Stocks et al. 2010a). Schwaiblmair (1997) reported one trea
(CTH) tment-r
Care.elated
adverse event that was self-limiting.
Aged
D
1982
YSPNOEA
released
Six studies reported results on dyspnoea (Table 20). Two RCTs rep
Act orted relative dyspnoea rates for
and
patients treated with A1PI and placebo (Chapman et al. 2015; Dirksen et al. 2009). Dyspnoea was
been
not reported in the EXACTLE trial publication, and was instead identified on clinicaltrials.gov. The
pooled rate across RCTs was higher in patients treated with A1PI (RR=1.23, 95% CI 0.63 to 2.4),
has
Health
noting that the pooled estimate was not statistical y significant, and was subject to moderate
of
heterogeneity (I2=60%, P=0.11). Two patients in the EXACTLE trial experienced dyspnoea, and one
Information
experienced severe dyspnoea, in the placebo arm; however, it is unclear if these were mutually
of
exclusive.
document
Three single arm studies reported rates of dyspnoea per patient, and a fourth reported the number
of dyspnoea events as a proportion of all adverse e
Department vents (Barker et al. 1997; McElvaney et al. 2017;
This Freedom
Stoller et al. 2003; Wencker et al. 1998). Single arm studies which reported a rate of patients
the
experiencing dyspnoea after AT showed dyspnoea had a median occurrence of 18.3% in the patient
population (range 3.8-34
by .8%). The total rates of dyspnoea are presented in Table 22. For more detail
on adverse events reported in the studies see Appendix D, Tables 101 & 102.
Table 22
Results of dyspnoea across the randomised control ed trials and single-arm studies
RCT ID
Risk of Follow-up Treatment dose / Intervention
Comparator
Relative difference
bias
frequency
rate (proportion) rate (proportion) RR (95% CI)
RAPID
Low
24 months 60mg/kg per week 17/93 (18.3%)
10/87 (11.5%)
1.59
(Chapman et
(0.77 to 3.28)
al. 2015)
EXACTLE*
Low
30 months 60mg/kg per week 0/38 (0.0%)
3/39 (7.7%)
0.15
(Dirksen et al.
(0.01 to 2.74)
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
53
Page 67 of 218
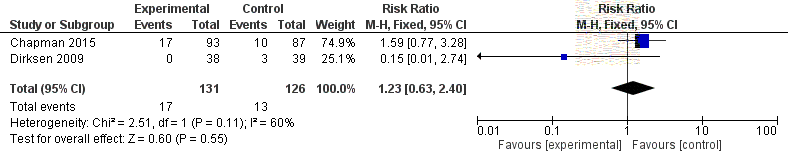
FOI 5155 - Document 4
2009)
Single arm Risk of Follow-up Treatment
Intervention
NA
NA
study ID
bias
dose/frequency rate (proportion)
Barker et al. High
4 months
120mg/kg every 2 8/23 (34.8%)
NA
NA
1997
weeks
RAPID-OLE Moderate 48 months 60mg/kg per week 28/140 (20.0%) NA
NA
(McElvaney et
al. 2017)
Stoller et al. Moderate 7 years
Unclear
61 events (8.5% NA
NA
2003
of all events)
Wencker et al. Moderate 6 years
60mg/kg per week 17/443 (3.8%)
NA
NA
1998
*Data obtained from results tab on www.clinicaltrials.gov.
Abbreviations:
CI = confidence interval,
NA = not applicable,
RCT = randomised controlled trial,
RR = relative risk.
the
under
(CTH) Care.
1982 Aged
Figure 10
Forest plot indicating rate of acute episodes of dyspnoea for A1PI
released
compared to placebo
Act and
DISCONTINUATION DUE TO ADVERSE EVENTS been
Ten studies, including two RCTs and eight single-arm studies, provided evidence on discontinuation
due to adverse events (Barker et al. 1994; Barros-Tizon et al. 2012; Campos et al. 2013; Chapman et
has
Health
al. 2015; Dirksen et al. 2009; Sandhaus et al. 2014; Stocks et al. 2010a; Stoller et al. 2003; The Alpha-
of
1-Antitrypsin Deficiency Registry Study Group 1998; Wenc
Information ker et al. 1998). Discontinuation due to
adverse events was rare in the included studies (Table 23).
of
Both RCTs demonstrated fewer patients discontinuing therapy in the A1PI arm, which is reflected in
document
the pooled estimate (RR=0.22, 95% CI 0.04 to 1.30) without any evidence of heterogeneity (I2,
P=0.94); however, this estimate includes the possibilit
Department y of no difference.
This Freedom
In the single arm studies, disco
the ntinuation had a median occurrence of 0.6% in the patient population
(range 0.0-7.1%). It is worth noting that discontinuation rates reported in the A1PI registry study
by
(1998) were higher due to lung transplantation (n=80/747, 10.7%), financial strain (n=16/747, 2.1%),
and unknown reasons (n=37/747, 5.0%). The forest plot for the meta-analysis of discontinuation due
to adverse events across the studies is presented in Figure 11.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
54
Page 68 of 218
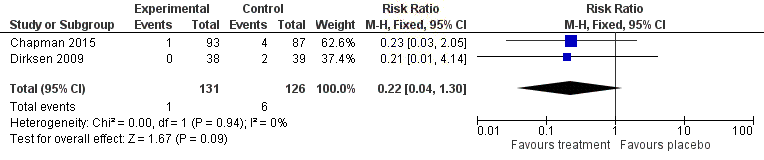
FOI 5155 - Document 4
Table 23
Results of discontinuation due to adverse events across the included randomised control ed trials
and single-arm studies
RCT ID
Risk of bias Follow-up Treatment
Intervention
Comparator
Relative difference
dose/frequency rate (proportion) rate (proportion) RR (95% CI)
RAPID
Low
24 months
60mg/kg per
1/93 (1.1%)
4/87 (4.6%)
0.23 (0.03 to 2.05)
(Chapman et
week
al. 2015)
EXACTLE
Low
30 months
60mg/kg per
0/38 (0.0%)
2/39 (5.1%)
0.21 (0.01 to
(Dirksen et al.
week
4.14)
2009)
Single arm Risk of bias Follow-up Treatment
Intervention
NA
NA
study ID
dose/frequency rate
(proportion)
the
The A1PI
Moderate
5 years
Unclear
4/747 (0.5%)
NA
NA
Deficiency
Registry
Study Group
under
1998
(CTH) Care.
Barker et al. High
48 months
60mg/kg every 1- 1/14 (7.1%)
NA
NA
1994
2 weeks
Barros-Tizón Moderate
18 months
60mg/kg every 0/127 (0.0%)
NA
NA
1982 Aged
et al.
1-3 weeks
released
2012
Act and
Campos et al. High
4 months
60mg/kg per
0/30 (0.0%)
NA
NA
2013
week been
Sandhaus et High
3 months
60mg/kg
2/50 (4.0%)
NA
NA
al. 2014
(frequency NR)
Health
Stocks et al. High
6 months
60mg/kg per
has 0/24 (0.0%) NA
NA
2010
week
of
Stoller et al. Moderate
7 years
Unclear
3/174 (1.
Information 7%) NA
NA
2003
of
Wencker et Moderate
6 years
60mg/kg per
3/443 (0.7%)
NA
NA
al. 1998
week
Abbreviations:
A1PI = alpha-1 proteinase inhibitor
document ,
CI = confidence interval,
NA = not applicable,
NR = not reported,
RCT = randomised
controlled trial,
RR = relative risk.
This Freedom
Department
the
by
Figure 11
Forest plot indicating discontinuation due to adverse events for A1PI compared to placebo
RAPID reported 1% discontinuation in the intervention group and 5% in the placebo group. EXACTLE
reported no discontinuations in the intervention group and 5% in the placebo group. Three single-
arm studies reported that no discontinuations occurred, while low levels were reported in the
remaining single-arm studies.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
55
Page 69 of 218
FOI 5155 - Document 4
HOSPITALISATION DUE TO ADVERSE EVENTS
Hospitalisation due to adverse events was reported, in four single-arm studies (Barker et al. 1994;
Schwaiblmair et al. 1997; Stoller et al. 2003; Wencker et al. 1998). No RCTs reported on this
outcome. The total rates of hospitalisation due to adverse events are presented in Table 24.
Reported rates were relatively low in three studies; and Schwaiblmair et al. (1997) reported no
patients were hospitalised due to adverse events. In addition to hospitalisation Stoller et al. (2003)
reported patients that experienced a physician visit or new medication due to adverse events
(21.1%). Hospitalisation had a median occurrence of 1.4% in the patient population (range 0.0-
14.3%).
the
Table 24
Hospitalisation due to adverse events across the included studies
Single-arm study ID
Risk of bias
Follow-up
Treatment dose/frequency
Event rate (proportion)
Barker et al. 1994
High
48 months
60mg/kg every 1-2 weeks
2/14 (14.3%)
under
Schwaiblmair et al. 1997
High
36 months
60mg/kg per week
0/20 (0.0%)
(CTH) Care.
Stoller et al. 2003
Moderate
7 years
Unclear
12/720 (1.7%)
Wencker et al. 1998
Moderate
6 years
60mg/kg per week
5/443 (1.1%)
1982 Aged
In Barker et al. (1994), one hospitalised patient had been hospitalised three times prior to
released
treatment, leading to the conclusion that this incident was du
Act e to continuation of pre-existing
and
disease. The other hospitalised patient experienced hypotension and respiratory distress. Stoller et
been
al. (2003) did not report reasons for hospitalisation for the 12 affected patients. In Wencker et al.
(1998), four hospitalised patients experienced anaphylactic reactions to the treatment; one
has
Health
experienced worsened congestive heart failure and related respiratory failure.
Information
of
ANY ADVERSE EVENTS
of
Sixteen studies, including all of the RCTs, provided evidence on any adverse events occurring after
treatment with the intervention (Bark
document er et al. 1997; Barker et al. 1994; Barros-Tizon et al. 2012;
Campos et al. 2013; Chapman et al. 2015; Dirksen et al. 1999; Dirksen et al. 2009; Hubbard and
Department
Crystal 1988; McEl
This vaney et al. 2017; Sandhaus et al. 2014; Schmidt et al. 1988; Schwaiblmair et al.
Freedom
1997; Stocks et al. 2010a; Stoller et al. 2003; Wencker et al. 1998; Wewers et al. 1987). Total rates of
the
adverse events are presented in Table 25. The rate of any patients experiencing any adverse event
by
ranged from 0%-100%, with a median of 37%. For more detail on adverse events reported in the
studies see Appendix D, Tables 101 & 102.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
56
Page 70 of 218
FOI 5155 - Document 4
Table 25
Results of any adverse events across the included randomised controlled trials and single-arm
studies
RCT ID
Risk of
Follow-up Treatment
Intervention
Comparator
Relative
bias
dose/frequency
Total (proportion) Total (proportion) difference
RR (95% CI)
RAPID
Low
24 months
60mg/kg per week 92/93 (98.9%)
86/87 (98.9%)
1.0 (0.97 to
(Chapman et
1.03)
al. 2015)
EXACTLE
Low
30 months
60mg/kg per week 37/38 (97.4%)
38/39 (97.4%)
1.0 (0.93 to
(Dirksen et al.
1.07)
2009)
DIRKSEN99 Low
36 months
250mg/kg per
0/28 (0.0%)
0/28 (0.0%)
NA
(Dirksen et al.
month
1999)
the
Single arm Risk of
Follow-up Treatment
Intervention
NA
NA
study ID
bias
dose/frequency
rate (proportion)
Barker et al. High
48 months
60mg/kg every 1-2 4/14 (28.6%)
NA
NA
1994
weeks
under Care.
Barker et al. High
4 months
120mg/kg every 2 21/23 (91.3%)
NA (CTH) NA
1997
weeks
Barros-Tizón Moderate 18 months
60mg/kg every 1-3 11/127 (8.7%)
NA
NA
et al.
weeks
1982 Aged
2012
released
Campos et al. High
4 months
60mg/kg per week 41/60 (68.3%)
NA
NA
Act and
2013*
Hubbard &
Moderate 12 months
250 mg/kg every
0/9 (0.0%)
NA
NA
been
Crystal 1988
28 days
RAPID-OLE Moderate 48 months
60mg/kg per week 138/140 (98.6%)
NA
NA
Health
(McElvaney et
has
al. 2017)
of
Sandhaus et High
3 months
60mg/kg
49/50 (98
Information .0%) NA
NA
al. 2014
(frequency NR)
of
Schmidt et al. High
6 months
60mg/kg per week 3/20 (15.0%)
NA
NA
1988
document
Schwaiblmair High
36 months
60mg/kg per week 1/20 (5.0%)
NA
NA
et al. 1997
Department
Stocks et al. High
6 months
60mg/kg per week 11/24 (45.8%)
NA
NA
This Freedom
2010*
the
Stoller et al. Moderate 7 years
Unclear
174/720 (24.2%)
NA
NA
2003
by
Wencker et Moderate 6 years
60mg/kg per week 65/443 (14.7%)
NA
NA
al. 1998
Wewers et al. High
6 months
60mg/kg per week 4/21 (19.0%)
NA
NA
1987
Abbreviations:
CI = confidence interval,
NA = not applicable,
NR = not reported,
RCT = randomised controlled trial,
RR = relative risk.
*The same patients at dif erent time points/intervention products.
The RAPID trial reported the same proportion of adverse events in the intervention and placebo
groups (98.9%). Events occurring in more than 10% of patients in the intervention group were:
infections/infestations, respiratory disorders, administration site issues, gastrointestinal disorders,
nervous system disorders, musculoskeletal disorders, nasopharyngitis, COPD, oropharyngeal pain,
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
57
Page 71 of 218
FOI 5155 - Document 4
cough, aggravation, lower respiratory disorders, dyspnoea, nausea, influenza, upper respiratory
disorders, pyrexia, bronchitis, sinusitis, back pain, and pneumonia (Chapman et al. 2015).
The EXACTLE trial also reported the same proportion of adverse events in the intervention and
placebo groups (97.4%). Events occurring in more than 5% of patients in the intervention group were
severe exacerbations, pneumonia, pneumothorax, and atrial fibrillation (Dirksen et al. 2009).
Patients in the DIRKSEN99 trial did not experience any adverse effects (Dirksen et al. 1999).
The RAPID-OLE trial stated that there were no safety concerns with the intervention. Adverse events
reported in an appendix to the paper were similar between intervention groups, both of which were
treated with open-label Zemaira at this stage of the RAPID trial.
the
A large disparity is noted between the RCTs and the observational studies in terms of number of
adverse events reported. In the single arm studies, the rate of any patients experiencing any adverse
event ranged from 0.0 to 100.0% with a median of 24.2%. This may indicate that adverse events
under Care.
have been under-reported in the observational studies. Adverse events reported most f
(CTH) requently in
the observational studies (in descending order of occurrence) were headache, fever, dyspnoea,
cough, nausea, and COPD exacerbation. These were mainly self-limiting with minimal medical
1982 Aged
attention required.
released
Act and
INFECTION
been
Products derived from human plasma may contain infectious viruses that cause disease. The risk of
infectious agents is reduced by screening blood donors for expo
Health sure to certain viruses, and by
has
inactivating or removing viruses during manufacture (Aventis Bering 2003). Seven studies took blood
of
tests pre- and post-intervention to test if infection with
Information human immunodeficiency virus (HIV),
Hepatitis B virus, Hepatitis C virus, and p
of arvovirus B19 had occurred during the treatment (Hubbard
and Crystal 1988; Schmidt et al. 1988; Schwaiblmair et al. 1997; Stocks et al. 2010a; Stoller et al.
2003; Wencker et al. 1998; Wewers
document et al. 1987). No case of infection was reported in any of the
studies. This Freedom Department
PROLASTIN-C AND ZEMAIRA SAFETY OUTCOMES
the
The specific products under assessment featured in four studies. Zemaira was the treatment given to
by
intervention patients (n=93) in the RAPID and RAPID-OLE trials (Chapman et al. 2015; McElvaney et
al. 2017). PROLASTIN-C was the treatment given to patients (n=54) in two single-arm studies
(Chapman et al. 2015; Stocks et al. 2010a). Rates of severe adverse events in these studies are
reported in Table 26.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
58
Page 72 of 218
FOI 5155 - Document 4
Table 26
Results of severe adverse events across the RCTs and non-control ed trials treating with Zemaira
and PROLASTIN-C
RCT ID
Risk of bias Follow-up Treatment
Intervention
Comparator
Relative
product
Total (proportion) Total (proportion) difference
RR (95% CI)
RAPID
Low
24 months
Zemaira
28/93 (30.1%)*
28/87 (32.0%)*
0.94 (0.61 to
(Chapman et
1.44)
al. 2015)
Single arm Risk of bias Follow-up Treatment
Intervention
NA
NA
study ID
product
rate (proportion)
Campos et al. High
4 months
PROLASTIN-C 0/30 (0.0%)
NA
NA
2013
RAPID-OLE Moderate
48 months
Zemaira
42/140 (30.0%)
NA
NA
(McElvaney et
the
al. 2017)
Stocks et al. High
6 months
Prolastin
2/24 (8.3%)
NA
NA
2010
PROLASTIN-C
Abbreviations:
CI = confidence interval,
RCT = randomised controlled trial,
RR = relative risk. under
* Data obtained from results tab on the NIH’s clinical trials website.
(CTH) Care.
In the RAPID trial, severe adverse events in both intervention (Zemaira) and control (placebo) groups
1982 Aged
was approximately 30% for both groups. Events occurring in more than 5% of patients were COPD in
released
the Zemaira group, and pneumonia and upper respiratory infectio
Act n in the p
and lacebo group (Chapman
et al. 2015).
been
In the RAPID-OLE trial, McElvaney (2017) reported that 30% of patients experienced serious adverse
has
Health
events for both groups.
Information
of
Campos et al. (2013) is a cross-over RCT comparing doses, which for the purposes of the safety
of
evaluation of A1PI versus Best Supportive Care only provides single-arm data to inform the research
question. As such, this has been treated as a single-arm study. The patient population (n=30) was
document
treated with PROLASTIN-C, and the study compared the standard dose (60mg/kg) to a double dose
(120 mg/kg). Safety outcomes were similar to those in the other included studies. No patients
This
Department
experienced severe adverse events
Freedom .
the
The study by Stocks et al. (2010), is also treated as a single-arm study. Half of the patients (n=24)
by
were treated with PROLASTIN-C and the other half with Prolastin. Patients in this study experienced
two serious adverse events thought to be related to the treatment (two cases of pruritus in one
patient after administration with PROLASTIN-C).
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
59
Page 73 of 218
FOI 5155 - Document 4
IS IT EFFECTIVE (RCT EVIDENCE)?
Summary – Is A1PI more effective than BSC or placebo?
Three RCTs investigated the clinical ef icacy of A1PI AT compared to placebo. CT-measured lung density was
the primary outcome in two RCTs, and FEV1 was the primary outcome in one RCT.
At 24-30 months there were no significant dif erences between A1PI AT and placebo in relation to mortality,
exacerbation of COPD, hospitalisation due to COPD exacerbation, quality of life, respiratory function (FEV1),
exercise capacity (incremental shut le walk test) or carbon monoxide dif usion (DLCO). No relevant data was
identified for dyspnoea as a measure of respiratory function, or the BODE index (BMI, obstruction, dyspnoea,
exercise capacity).
the
The only statistically significant difference that was observed was for CT-measured lung density, which favoured
AT. The clinical significance of this difference is uncertain, however, as MCIDs for changes in CT-lung density
under
have not been established in the literature.
(CTH) Care.
MORTALITY
Aged
released 1982
Randomised control ed trials
Act and
One RCT, with 180 patients fol owed for 24 months, investigated relative mortality rates of A1PI and
placebo or no treatment (Chapman et al. 2015). One
been patient died of respiratory failure in the A1PI
group, and three patients died in the placebo group due to sepsis, pneumonia, and metastatic breast
has
Health
cancer. Due to the smal number of events in each group, the calculated relative difference reported
of
in Table 27 is subject to error and should be interpreted with caution. Based on the identified data,
Information
the estimated relative survival gain within 24 months is highly uncertain. The DIRKSEN99 study
of
reportedly collected data on mortality but this was not reported.
document
Table 27
Results of mortality across the randomised control ed trials at 24 months
Study ID
Risk of bias
Follow-up
Intervention
Comparator
Relative difference
This Freedom
Department
n with event/N (%) n with event/N (%)
RR (95% CI)
RAPID
Low the 24 months
1/93
3/87
0.31
(Chapman et al.
(1.1%)
(3.4%)
(0.03 to 2.94)
2015)
by
Abbreviations:
CI = confidence interval,
RR = risk ratio.
The RAPID trial also reported a “terminal event” as a composite endpoint of progressive emphysema
(Chapman et al. 2015). A terminal event was defined as lung transplantation or mortality. Five
patients experienced a terminal event. For these patients, the average lung density at terminal event
was 19.0 g/L (95% CI 3.5 to 29.5). The study authors then calculated expected life years gained, by
working out the difference between lung density of the whole sample at the baseline (47.1 g/L, 95%
CI 23.0 to 76.1) and the averaged lung density at terminal events, and then this difference was
divided by the annualised rate of lung density decline. Based on this estimate, the authors calculated
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
60
Page 74 of 218
FOI 5155 - Document 4
an interpolated survival gain of 18.1 years (12.2 to 30.1) for A1PI patients, compared to 12.3 years
(95% CI 8.1 to 19.9) in the placebo group. The internal validity of this estimate is questionable, due
to the low number of terminal events. Further, this calculation assumes a linear progression of lung
density decline, which is uncertain based on the available trial data.
Non-randomised control ed trials
Two studies investigated the relative mortality rate in A1PI deficient patients undergoing
augmentation therapy or not (Table 28). The studies comprised a total of 1091 patients, who were
followed for an average of 42–52 months (Tonel i et al. 2009; Alpha-1-Antitrypsin Deficiency Registry
Study Group 1998).
the
The Alpha-1-Antitrypsin Deficiency Registry Study Group performed a retrospective analysis of
registry data from March 1989 to October 1992. There were 147 deaths, the cause of which could be
identified in 118 deaths. Emphysema (n = 85) and cirrhosis (n = 12) were the predominant causes of
under
death. There were 16 other causes of death which comprised one to three patients. Morali
Care.ty risk
(CTH)
was significantly lower for patients receiving AT compared to those not receiving AT when adjusted
for age, education and initial FEV1 % predicted (RR 0.64, 95% CI 0.43 to 0.94,
p = 0.02). It was further
1982 Aged
observed that the effect of augmentation therapy on mortality varied according to FEV1 % predicted.
released
For patients with FEV1 < 35% or ≥ 50% there was no effect of augmentation therapy (
p = 0.44 and
Act and
0.64 respectively). However, for patients with a FEV1 between 35% and 49%, augmentation was
associated with a reduced risk of mortality (
p < 0.00
been 1). The results of this study were not adjusted
for differences in baseline socioeconomic status, smoking status or co-morbidities.
has
Health
Tonelli et al. (2009) conducted a retrospective analysis
of of the Alpha-1 Foundation DNA and Tissue
Information
bank. Reported 5-year mortality rates were 4.0% for A1PI augmentation and 2.5% (P = 0.58) in non-
of
augmented patients respectively; however, it was unclear how many patients were included in each
arm of the analysis (i.e. how many had 5-year follow-up data). These were adjusted for in the
document
analysis. Socioeconomic status was not reported, but is a recognised confounding domain.
Department
Table 28
Results of mortality across the non-randomised controlled trials
This Freedom
Study ID
Follow- Risk of Patient population
Intervention
Comparator
Relative difference
the
up
bias
n with event/N (%) n with event/N (%)
RR (95% CI)
Median
by
(range)
Alpha-1-
Antitrypsin
52 (12
Deficiency
– 86)
High
Partly receiving
33/261 (12.6%)
Registry Study months
Always receiving
46/389 (11.8%)
24/277 (8.7%)
NR
Group 1998
Tonelli et al.
41.7
2009
(2.6)
High
NR (4.0%)
NR (2.5%)
NR
months
Abbreviations:
CI = confidence interval,
NR = not reported,
RR = risk ratio,
SD = standard deviation.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
61
Page 75 of 218
FOI 5155 - Document 4
EXACERBATION OF COPD
Randomised control ed trials
Two RCTs reported exacerbations of COPD at 24 months, with a total of 257 patients (Table 29)
(Chapman et al. 2015;Dirksen et al. 2009). Data were not meta-analysed, as the distributions were
not normal y distributed in the EXACTLE trial. The RAPID trial presented as an adjusted risk ratio (RR
= 1.26, 95% CI 0.92 to 1.74) from a negative binomial regression model, in which country and
treatment were fixed effects, and adjustment was made for treatment duration. The EXACTLE trial
reported an annualised mean difference of 0.36 (95% CI -0.44 to 1.16). In both studies, patients
treated with A1PI experienced a greater number of exacerbations; however, this difference was not
statistically significant.
the
Table 29
Results of exacerbations across the direct randomised control ed trials
Study ID
Follow-up Risk of
Intervention
Comparator
Absolute or relative
under
bias
Mean annual number Mean annual number
difference
(CTH) Care.
± SD or 95% CI
± SD or 95% CI
MD or RR (95% CI)
RAPID
24
(Chapman et al. 2015) months
Low
1.7 (1.51 to 1.89)
1.42 (1.23 to 1.61)
RR = 1.26 (0.92 to
1.74)
1982 Aged
EXACTLE
<30
released
(Dirksen et al. 2009)
months
Low
2.55 ± 2.14
2.19 ±1.33
MD = 0.36 (-0.44 to
1.16)
Act and
Abbreviations:
CI = confidence interval,
MD = mean dif erence,
RR = relative risk,
SD = standard deviation.
Non-randomised control ed trials
been
One study reported the number of COPD exacerbations in 127 pat
Health ients 18 months before, and 18
has
months after commencing augmentation therapy (Barros-Tizon et al. 2012). There was a significant
of
difference in the number of exacerbations (Table 30) an
Information d the per cent of patients experiencing
exacerbations fol owing augmentation th
of erapy (59.1 vs 44.1% (before and after respectively)
p <
0.005). However, the magnitude of the effect was relatively smal , with the mean number of
exacerbations decreasing by 0.2 per
document patient. Furthermore, the number of severe exacerbations did
not differ significantly between the two groups. The authors noted that the multivariate analysis was
Department
likely biased favourin
This g the use of augmentation therapy.
Freedom
the
Table 30
Results of exacerbations across the non-randomised trials
by
Study ID
Follow-up
Risk of bias
Intervention
Comparator
Mean number ± SEM
Mean number ± SEM
Barros-Tizon et al. 2012
36 months
High
1.2 ± 1.6
1 ± 2.2*
Abbreviation:
SEM = standard error of mean.
p < 0.01
HOSPITALISATION DUE TO COPD EXACERBATIONS
The RAPID and EXACTLE trials recorded hospitalisation rates due to exacerbations of COPD at 24 and
30 months respectively (Chapman et al. 2015; Dirksen et al. 2009). Data were not meta-analysed due
to the difference in follow-up duration across studies. Both trials reported the overall number of
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
62
Page 76 of 218
FOI 5155 - Document 4
patients requiring hospitalisation due to an exacerbation of COPD, however, the total number of
events experienced and hospitalisation days were not reported consistently. The estimated relative
differences showed conflicting directions of effect across the included studies; however, neither
study demonstrated a statistically significant difference between treatment groups. Across the two
studies, three patients had two, three, and more than three hospitalisations in the treatment arm; in
the comparator arm one patient had two and three patients had three hospitalisations. A summary
of results of hospitalisations across the direct randomised controlled trials is presented in Table 31.
Table 31
Results of hospitalisations across the direct randomised control ed trials
Study ID
Risk of bias
Follow-up
Intervention
Comparator
Relative difference
n with event/N (%) n with event/N (%)
RR (95% CI)
RAPID*
the
(Chapman et al.
High
24 months
13/93 (14.0%)
9/87 (10.3%)
2015)
patients
patients
1.35 (0.61 to 3.00)
EXACTLE
under
(Dirksen et al.
Low
<30 months
6/38 (15.8%)
11/39 (28.2%)
(CTH) Care.
2009)
patients
patients
0.56 (0.23 to 1.36)
Abbreviations:
CI = confidence interval,
N = total number of patients.
* Results from the RAPID trial were sourced from clinicaltrials.gov.
1982 Aged
QUALITY OF LIFE
released
Act and
Randomised control ed trials
been
Quality of life was reported in two of the included RCTs, with a total sample size of 248 patients
(Chapman et al. 2015; Dirksen et al. 2009). The individual results of each study are presented in
has
Health
Table 32. The forest plot for the meta-analysis of QoL is presented in Figure 12, showing no evidence
of
of heterogeneity. The primary studies and pooled estimate
Information showed a slightly lower increase in SGRQ
score at 24/30 months, corresponding to a slightly slower decline in QoL. This favours A1PI,
of
however, this difference was not statistically significant.
document
Table 32
Results of quality of life across the direct randomised control ed trials†
Department
Study ID
Follow-up Risk of
Intervention
Comparator
Mean difference (95%
This Freedom
bias Mean change ± SD Mean change ± SD
CI)
the
RAPID
24
(Chapman et al. 2015) months
Low
1.4 ± 11.1
2.2 ± 11.7
-0.80 (-4.14 to 2.54)
by
EXACTLE
<30
(Dirksen et al. 2009)
months
Low
1.48 ± 9.24*
2.37 ± 9.24*
-0.89 (-5.28 to 3.50)
Abbreviations:
CI = confidence interval,
SD = standard deviation.
† Quality of life was measured using the St Georges Respiratory Questionnaire, which measures quality of life related to obstructive
airway disease on a scale from 0 to 100, with higher scores indicating greater limitations.
* An average SD for both study arms was calculated from available p scores.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
63
Page 77 of 218
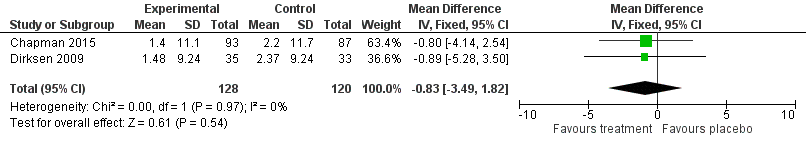
FOI 5155 - Document 4
Figure 12
Forest plot indicating mean changes in St George’s Respiratory Questionnaire results for A1PI
compared to placebo
Non-randomised control ed trials
Health-related quality of life was reported in one non-RCT examining patients from the German
multicentre COPD cohort COSYCONET (Karl et al. 2017). A total of COPD patients (n=106) and
without (n=25) A1PI augmentation were compared. The analysis was adjusted for GOLD grade, age,
the
gender, smoking status, education, and body mass index. It was unclear at what time point health-
related quality of life was measured. Overall, there were no significant differences in health-related
quality of life when assessed by CAT, SGRQ or ED-5Q-3 L in COPD, AATD and AATD patients receiving
under Care.
augmentation therapy. However, significant differences were found in SGRQ and EQ-5
(CTH) D VAS scores
when comparing AATD patients who received augmentation therapy to those who did not (
p < 0.05).
1982 Aged
Lieberman (2000) indirectly assessed quality of life in an internet survey of AATD patients. Eighty
released
three, fourteen and three per cent of patients reported a benefit, unknown and no benefit fol owing
Act and
augmentation therapy respectively. The perceived benefit from augmentation therapy was
attributable to the reduction in number of lung infe
been ctions. However, it is unclear whether patient
demographics differed among the groups. The results in each non-randomised controlled trial are
Health
given in Table 33.
has
Information
of
Table 33
Results of quality of life across the non-randomised controlled trials†
of
Study ID
Risk of bias Quality of life
Intervention
Comparator
Mean difference (95% CI)
measure
Mean change ± SD Mean change ± SD
document
or n (%)
or n (%)
Karl et al. 2017 Serious
SGRQ
46.6 ± 16.4
37.5 ± 20.2
-9.1 (-16.7, -1.6)*
CAT
18.9 ±
Department 6.6
17.2 ± 7.3
-1.7 (-4.7, -1.3)
This
EQ-5D-3 L
Freedom
83.0 ± 19.1
83.9 ± 19.4
0.9 (-7.5, 9.3)
utility
54.4 ± 18.8
63.6 ± 18.8
9.2 (0.9, 17.5)*
the EQ-5D VAS
by
Lieberman 2000 Serious
Perceived
74/89 (83%)
NR
NR
benefit
12/89 (14%)
No benefit
3/89 (3%)
Did not know
Abbreviations:
CAT = COPD assessment questionnaire,
CI = confidence interval,
EQ-5D-3 L = Euroqol group 5 domain questionnaire,
EQ-5D VAS = Euroqol group 5 domain questionnaire including visual analogue scale,
NR = not reported,
SD = standard deviation,
SGRQ
= St George Respiratory Questionnaire.
*p < 0.05. mean dif erence was significant between AT versus no AT.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
64
Page 78 of 218
FOI 5155 - Document 4
EXERCISE CAPACITY
One RCT investigated exercise capacity, as measured by incremental shuttle walk distance at 24
months (Chapman et al. 2015). The results are presented in Table 34. Due to reporting limitations, it
is unclear whether the change in walking distance (i.e. results showing an increase in walking
distance), or total walking distance (i.e. results showing a severe reduction in walking distance) at 24
months was reported. Based on data entered into clinicaltrials.gov it appears to be the change in
exercise capacity, in which case both groups reported an increase in exercise capacity at 24 months.
Nevertheless, the mean difference in exercise capacity was not statistically significant between
treatment groups.
Table 34
Results of shut le walk distance (metres) in the direct randomised controlled trial
the
Study ID
Follow- Risk of Intervention Intervention Comparator Comparator Mean difference
up
bias
baseline
24 months
baseline
24 months
(95% CI)
mean ± SD
mean ± SD
mean ± SD
mean ± SD
under
RAPID
Care.
24
10.8 ±
435.1 ±
(CTH)
(Chapman et al.
2015)
months Low
424.5 ±
183.0
139.8
199.7
16.1 ± 101.6
-13.09 (NR)
p = 0.48*
Abbreviations:
CI = confidence interval,
NR = not reported,
SD = standard deviation.
*Adjusted for country, treatment group, and baseline values.
Aged
released 1982
DYSPNOEA
Act and
None of the identified RCTs evaluated dyspnoea as a functional outcome. Instead, the RCTs reported
been
acute episodes of dyspnoea as adverse events (see Section B.6 Dyspnoea).
has
Health
RESPIRATORY FUNCTION: CHANGE IN FEV1 (ML OR % PREDICTED)
Information
of
Randomised control ed trials
of
FEV1 was reported variably across the three included RCTs as either FEV1 (mL) or FEV1 % predicted,
and as either an annualised differenc
document e, or an overall difference at last follow-up. Standardised mean
differences were calculated in order to pool FEV1 outcomes from the three studies. Data from the
Department
pooled analysis are reported in Table 35 and Figure 13.
This Freedom
the
The analysis for this report included two studies that reported changes in FEV1 (mL) (Dirksen et al.
1999; Dirksen et al. 2009
by ), and one study that reported a change in FEV1 % predicted (Chapman et al.
2015). The analysis is similar to that conducted in the Cochrane review (Gotzsche and Johansen
2016), with the exception of denominators for the RAPID trial—the analysis for this report included
the reported ITT population, whereas the Cochrane review reported the per-protocol analysis minus
three patients without CT lung density scan data.
The forest plot for the meta-analysis of FEV1 (Figure 13) shows no evidence of heterogeneity. FEV1,
measured by both mL and % predicted, declined in both treatment arms across the included RCTs.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
65
Page 79 of 218
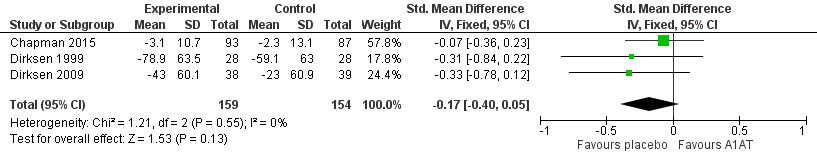
FOI 5155 - Document 4
The reported extent of decline in FEV1 was lower in patients treated with placebo, however, the
estimated differences were not statistically significant.
Table 35
Results of change in FEV1 (% predicted or mL) across the direct randomised control ed trials†
Study ID
Follow-up Risk of
Intervention
Comparator
Std. mean difference
bias
mean ± SD
mean ± SD
(95% CI)
RAPID
24
(Chapman et al. 2015) months
Low
-3.1% ± 10.7%
-2.3% ± 13.1%
-0.07 (-0.36 to 0.23)
EXACTLE*
<30
(Dirksen et al. 2009)
months
Low
-43mL ± 60.1 mL
-23mL ± 60.9mL
-0.33 (-0.78 to 0.12)
DIRKSEN99
>36
(Dirksen et al. 1999)
months
High
-78.9mL ± 63.5mL
-59.1mL ± 63 mL
-0.31 (-0.84 to 0.22)
Abbreviations:
CI = confidence interval,
SD = standard deviation.
the
† FEV1 is a measure of the amount of air a person can forcibly expire in one second, with lower mL and % predicted values indicating
more severe lung disease. A decline in FEV1 mL or % predicted represents a worsening of lung function.
* EXACTLE also reported % predicted. Changing the analysis to include FEV1 % predicted for this study instead of FEV1 mL does not
change the result.
under
(CTH) Care.
Aged
released 1982
Act and
Figure 13
Forest plot indicating standardised mean differnces in FEV1 for A1PI compared to placebo
been
Non-randomised control ed trials has
Health
The annual change FEV1 (mL/year) was reported in five studies corresponding to 1609 patients
of
(Barros-Tizon et al. 2012, Tonelli et al. 2009, Wencker et
Information al. 1998, Alpha-1-Antitrypsin Deficiency
Registry Study Group 1998, Seersholm et al. 1997) (Table 36). Two studies compared the effects of
of
augmentation within the same patient population (pre- post-intervention design) (Barros-Tizon et al.
2012, Wencker et al. 1998). Three
document studies retrospectively analysed registry data and compared
patients who received augmentation to those who did not (Tonelli et al. 2009, Alpha-1-Antitrypsin
Department
Deficiency Registry
This Study Group 1998, Seersholm et al. 1997). Different methodologies were used to
Freedom
measure FEV1. For example, some studies recorded FEV1 measurements before and after
the
bronchodilator use, while others only recorded after. Further, the type of analysis and the covariates
by
adjusted for in each study differed.
The largest non-randomised study concluded there was no statistical difference in annual FEV1
decline between patients who were treated with and not treated with augmentation therapy (
p =0.4) after correcting for gender, smoking status, age, bronchodilator responsiveness and FEV1 %
predicted in a multivariate analysis (Alpha-1-Antitrypsin Deficiency Registry Study Group 1998, n =
927). However, the smaller trials (Barros-Tizon et al. 2012, Tonelli et al. 2009, Wencker et al. 1998
and Seersholm et al. 1997) al reported a significant difference between the augmentation therapy
and no augmentation therapy groups (
p < 0.05, = 0.05, 0.02 and 0.02 respectively). Differences in the
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
66
Page 80 of 218
FOI 5155 - Document 4
patient population (and thus the medical strategy used to treat AATD), the method of analysis and
the co-variates adjusted for, likely underscore the difference between the differing results studies.
Interestingly, the effects of augmentation therapy appeared most pronounced in patients with a
predicted FEV1 of 30–65% and >65%. Two studies found significant group differences in patients with
FEV1 between 30–65% (Alpha-1 registry study group 1998, and Seersholm et al. 1997,
p = 0.03 and
0.04 respectively) and FEV1 > 65% (Tonelli et al. 2009, Wencker et al. 1998,
p < 0.01 and 0.05
respectively). In all studies, there was no statistical difference between augmentation therapy and
the no augmentation therapy group when FEV1 was ≤ 30%.
Table 36
Results of change in FEV1 (% predicted or mL) across the non-randomised controlled trials
Study ID
Risk of Patient population
Intervention
Comparator
M
the
ean difference
bias
Mean change ± Mean change ± SEM
(95% CI)
SEM
Barros-Tizon et al.
under
2012
Serious
Al patients
#1.25 ± 0.5
1.19 ± 0.5*
-0.06 (-0.18, 0.06)
(CTH) Care.
All subjects
10.6 ± 21.4
37.0 ± 12.1*
26.4 (-49.4, 102.2)
FEV
0.9 ± 17.6
20.1 ± 31.1
19.2 (-97.1, 135.6)
Tonelli et al. 2009
Serious
1 < 30%
FEV1 30 – 65 %
2.08 ± 24.0
-51.9 ± 18.1
49.9 (-85.5, 185.1)
1982 Aged
FEV1 > 65%
-108.7 ± 17.3
-29.2 ± 15.3**
-79 (-128.5, -30.5)
released
All subjects
#-49.2 ± 60.8
-34.3 ± 29.7*
14.9 (1.3, 28.5)
Act and
FEV1 < 30%
-15.3 ± 38.5
-19.0 ± 18.0
-3.7 (-20.8, 13.4)
Wencker et al. 1998
Serious
FEV1 30 – 65%
-49.3 ± 43.4
-37.8 ± 25.0
11.5 (-1.3, 24.3)
been
FEV1 > 65%
-122.5 ± 108.4
-48.9 ± 54.9*
73.6 (-2.8, 150.0)
Al patients
-51.8 ± 2.7
-56.0 ± 3.8
4.2 (-5.7, 14.2)
has
Health
Alpha-1-Antitrypsin
FEV1 < 35%
-43.9 ± 3.4
-46.5 ± 6.2
2.6 (-11.3, 16.5)
Deficiency Registry
Serious
FEV
of
1 35 – 49%
-66.4 ± 5.0
-93.2 ± 11.1
26.8 (2.8, 50.9)*
Study Group 1998
FEV
Information
1 50 – 79%
-73.7 ± 6.8
-81.2 ± 8.9
7.5 (-14.7, 29.6)
FEV1 ≥ 80%
-63.0 ± 12.8
-39.2 ± 5.6
-23.8 (-50.9, 3.3)
of
All subjects
#53.0 ± 37.6
74.5 ± 59.6*
21.5 (-112.3, 155.3)
Seersholm et al. 1997 Serious
FEV1 ≤ 30%
24.2 ± 23.3
30.9 ± 36.3
6.7 (-82.6, 96.0)
document
FEV1 31 – 65%
61.8 ± 25.3
82.8 ± 49.3*
21.0 (-77.5, 119.5)
FEV1 > 65%
162 ± 28.7
140 ± 83.2
-22.0 (-212.0, 168.0)
Department
Abbreviations:
CI = confidence interval,
FEV1 = forced expiratory volume in 1 second,
SEM = standard error of mean,
SD = standard
This Freedom
deviation.
# data presented as SD not SEM. the
*
p≤ 0.05, **
p < 0.001.
by
CT-MEASURED LUNG DENSITY
CT lung density decline was the primary outcome in two of the included RCTs (Chapman et al. 2015;
Dirksen et al. 2009) and a secondary outcome in the third (Dirksen et al. 1999). The results of the
individual studies are presented in Table 37. The EXACTLE trial reported four methods for measuring
CT lung density. We used the 24-month data from the first method (physiological adjustment), as
both the DIRKSEN99 and RAPID trials used a similar method of physiological adjustment. The
Cochrane review included an average of the four methods, which yielded almost identical results
(MD 0.86, 95% CI 0.31 to 1.42) to the current meta-analysis review (Gotzsche and Johansen 2016).
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
67
Page 81 of 218
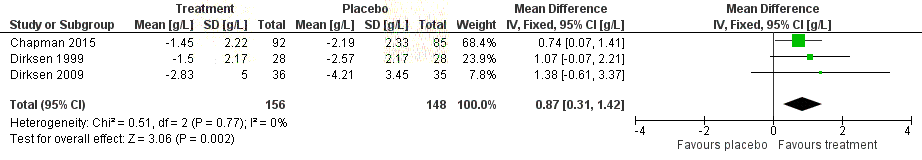
FOI 5155 - Document 4
The results of the meta-analysis are presented in Figure 14. The pooled estimate demonstrated a
slower rate of decline in CT-measured lung density across the included studies for A1PI patients (MD
0.87, 95% CI 0.31 to 1.42), with no evidence of heterogeneity (Chi2=0.51, I2=0%, P=0.78). The clinical
significance of this result is difficult to ascertain, as discussed in Section B.5.
Table 37
Results of CT-measured lung density (total lung capacity, g/L per year) across the direct randomised
controlled trials
Study ID
Risk of bias
Intervention
Comparator
Mean difference and 95%
mean ± SD
mean ± SD
CI
RAPID
(Chapman et al.
Low
-1.45 ± 2.20
-2.19 ± 2.30
0.74 (0.07 to 1.41)
2015)
EXACTLE
the
(Dirksen et al.
Low
-2.83 ± 5.01
-4.21 ± 3.45
1.38 (-0.63 to 3.39)
2009)
DIRKSEN99*
under
(Dirksen et al.
High
-1.50 ± 2.17
-2.57 ± 2.17
1.07 (-0.07 to 2.221)
(CTH) Care.
1999)
Abbreviations:
CI = confidence interval,
SD = standard deviation.
*Whole lung CT, g/L
Aged
released 1982
Act and
been
Health
Figure 14
Forest plot indicating changes in C
has
T-measured lung density (g/mL) in A1PI compared to placebo
measured at 24 to 30 months fol ow-up. (Chapm
of
an 2015 and Dirksen 1999 reported an annualised
rate, whereas Dirksen 2009 reported the change from baseline at 24 months.)
Information
C
of
ARBON MONOXIDE DIFFUSING CAPACITY
Randomised control ed trials
document
All three RCTs investigated carbon monoxide diffusing capacity (DLCO) (Chapman et al. 2015; Dirksen
This
Department
et al. 1999; Dirksen et al. 2009). Res
Freedom ults for each study are presented in Table 38 and the forest plot
is presented in Figure 15. Diffe
the rent units of measurement were used across studies, therefore
standardised mean differences were calculated in order to pool the results. Across the included
by
RCTs, DLCO deteriorated to a greater extent in the A1PI patients. This favoured placebo, however, the
difference was not statistically significant. There was no evidence of heterogeneity in the pooled
estimate (Chi2 = 0.66, I2 = 0%, P = 0.72). This analysis produced almost identical results to that
conducted in the Cochrane review (standardised mean difference (SMD) -0.11, 95% CI -0.35 to
0.12), as it utilised the same primary data and analytical method review (Gotzsche and Johansen
2016). The only difference was in the reported population size, whereby we included the ITT
population of the RAPID trial as reported in the manuscript, and the Cochrane review included the
per-protocol population minus patients without CT scan data (A1PI n=83, placebo n=67).
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
68
Page 82 of 218
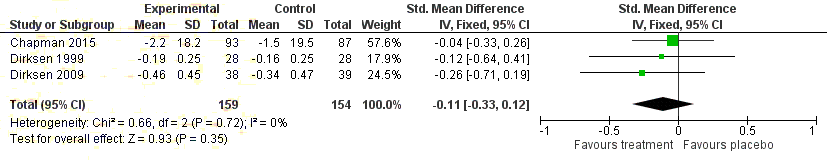
FOI 5155 - Document 4
Table 38
Results of carbon monoxide dif using capacity across the direct randomised controlled trials
Study ID
Risk of bias
Intervention
Comparator
Standardised mean
mean ± SD
mean ± SD
difference (95% CI)
RAPID
A
(Chapman et al. 2015)
Low
-2.2 ± 18.2
-1.5 ± 19.5
-0.04 (-0.34 to 0.26)
EXACTLE
B
(Dirksen et al. 2009)
Low
-0.46 ± 0.45
-0.34 ± 0.47
-0.14 (-0.67 to 0.38)
DIRKSEN99
C
(Dirksen et al. 2009)
High
-0.19 ± 0.25
-0.16 ± 0.25
-0.26 (-0.71 to 0.19)
the
Abbreviations:
CI = confidence interval,
SD = standard deviation.
A DLCO measured in mL/mm Hg per min, %
B DLCO measured in mmol/min/kPa
C DLCO measured in mmol/min/kPa/L
under
(CTH) Care.
Aged
released 1982
Act and
been
Figure 15
Forest plot indicating the standardised mean dif erence in carbon monoxide diffusing capacity (DLCO)
for A1PI compared to placebo
has
Health
Non-randomised control ed trials
Information
of
One study compared DLCO in patients before and after they had received augmentation therapy
of
(Barros-Tizon et al. 2012). Results are presented in Table 39.There was no statistical difference
between the two groups. The authors further compared DLCO to a normal healthy Spanish population
document
adjusted for age, sex, height and weight. There were no differences in the decrease of DLCO between
the healthy population and the study population. The authors reported this comparison narratively
This Freedom
Department
and did not show the precise data or the statistical tests used to assess group differences.
the
Table 39
Results of carbon monoxide dif using capacity across the non-randomised controlled trials
by
Study ID
Risk of Patient population
Intervention
Comparator
Mean difference
bias
Mean change ± SD Mean change ± SD
(95% CI)
Barros-Tizon et al.
2012
Serious Al patients
69.1 ± 69.2
58.9 ± 26.3
-10.2 (-23.1, 2.7)
Abbreviations:
CI = confidence interval,
SD = standard deviation.
BODE INDEX FOR COPD SURVIVAL PREDICTION
No evidence was identified that investigated the BODE index in AT patients compared to BSC or
placebo.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
69
Page 83 of 218
FOI 5155 - Document 4
Non-randomised control ed trials
Lieberman (2000) assessed the rate of self-reported lung infections in 89 and 47 AATD patients who
had and had not received augmentation therapy for > 1 year respectively (Table 40). Within-
patients, there was a significant difference in the number of lung infections after receiving
augmentation therapy compared to before (
p < 0.001). Additionally, there was significant
differences between patients who received augmentation therapy to those who did not (
p < 0.001).
However, the analysis did not adjust for differences in patient demographics such as smoking status,
comorbidities and concurrent medical treatments.
Table 40
Results of lung infections in non-randomised control ed trials
Study ID
Risk of bias Quality of life measure Before intervention After intervention Never received
the
N (%)
N (%)
intervention N (%)
Lieberman 2000
Serious
Lung infection < 2
27/89 (30%)*
73/89 (82%)
21/47 (45%)*
Lung infection ≥ 2
62/89 (70%)*
16/89 (18%)
26/47 (55%)*
p < 0.001 when compared to after intervention.
under
(CTH) Care.
AVERAGE HOSPITALISATION
Aged
Non-randomised control ed trials
released 1982
Two studies reported the average hospital stay between patients receiving and not receiving
Act and
augmentation therapy (Karl et al. 2017; Barros-Tizon et al. 2012) (Table 41). In general, patients who
received augmentation therapy reported less time sp
been ent in hospital compared to patients who did
not receive augmentation therapy. The difference was less than a day and statistical analysis
has
Health
comparing the two groups were not performed in both studies.
Information
of
Table 41
Results of hospitalisation days in non-randomised controlled trials
of
Study ID
Risk of bias
Intervention
Comparator
Mean difference (95% CI)
mean ± SD
mean ± SD
document
Karl et al. 2017
Serious
2.2 ± 5.7
2.7 ± 6.3
0.5 (-2.1, 3.1)
Barros-Tizon et al. 2012
Serious
3.9 ± NR
3.0 ± NR
NR
Department
Abbreviations:
CI = confidence interval,
NR = not reported,
SD = standard deviation.
This Freedom
B.7.
E
the
XTENDED ASSESSMENT OF HARMS
by
The search strategy used to identify post-marketing harms is documented in Appendix B. Searches
were targeted to identify any warning or recalls issued by the medical device and intervention
regulating authorities of Australia, New Zealand and the United States. In addition, the product
information sheets provided by the manufacturers of Zemaira and PROLASTIN-C (CSL Behring and
Grifols Therapeutics, respectively) were reviewed for any concerns not identified elsewhere. In both
documents it was noted that the therapies are contraindicated for patients with a known sensitivity
to A1PI products, and patients with immunoglobulin A (IgA) deficiency and antibodies against IgA.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
70
Page 84 of 218
FOI 5155 - Document 4
Post-market adverse drug reactions to PROLASTIN-C are reported as occasional flu-like symptoms,
allergic-like reactions, dyspnoea, tachycardia, shortness of breath, bronchospasm, wheezing,
urticaria, back pain, clamminess, sweating, diarrhoea, and fatigue. Less frequently hypotension,
anxiety, cyanosis, swel ing of hands and feet, angio-oedema, facial and lip oedema, nasal congestion,
sinusitis, abdominal pains or cramps, pallor, and weakness have also been reported. Cases of
transient increase in blood pressure or hypertension and chest pain have also been reported, but
these were rare Karl et al. (2017). Numbers of patients experiencing these events were not reported.
No post-marketing data on long-term adverse event rates was identified for Zemaira (2003), perhaps
due to the more recent entry of this product to the market.
B.8.
INTERPRETATION OF THE CLINICAL EVIDENCE
the
It is important to classify the therapeutic profile of AT in relation to BSC or placebo, that is, whether
it is therapeutically superior, inferior or equivalent to the comparator.
under
(CTH) Care.
On the basis of the evidence profile (summarised in Table 42),
it is suggested that, relative to BSC,
AT has inferior safety and uncertain effectiveness; however, relative to placebo, there were no
1982 Aged
important differences in safety outcomes. A summary of the clinical evidence from the
released
observational studies is provided in Appendix D, Table 106. Act and
Seventeen trials were available for safety outcomes, o
been f which three studies were placebo-controlled
RCTs (level II), four were non-randomised studies comparing doses of AT (Level III-II), and 10 were
has
Health
single-arm studies of AT (Level IV). The quality of the single-arm trials was poor, with most appraised
of
as having a high risk of bias. Overal , the populations in the included evidence base had good
Information
applicability to the proposed population in Australian practice, noting that the study populations
of
were largely Caucasian.
document
The conclusion of inferior safety is predicated on the understanding that the intervention is
proposed as an additional intervention to BSC, carrying a small risk of severe adverse events. It
This
Department
should be noted that most adverse
Freedom events associated with the intervention were mild, and severe
adverse events were not signific
the antly different across treatment and placebo arms in the RCTs. The
direct RCTs did not demonstrate a significant difference in severe adverse events, or discontinuation
by
due to adverse events between treatment groups. Further, no treatment-related deaths were
identified in any of the included studies.
Three direct RCTs evaluated the relative effectiveness of AT compared to placebo, with a total of 313
randomised patients. The overall quality of the included RCTs was mostly good, with the exception
of DIRKSEN99 which was poorly reported. The key uncertainties around the clinical trials were in
relation to allocation concealment, and the key risks of bias were in relation to conflicts of interest of
the study authors. Al of the RCTs were supported by industry. The key trials note a lack of power to
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
71
Page 85 of 218
FOI 5155 - Document 4
detect a change, which is accurate, however, power calculations are based on an assumption of an
estimated effect direction and size.
The only significant treatment effect observed was for CT-measured lung density. Al other
effectiveness outcomes reported non-significant differences. Minimum clinically important
differences for the primary outcome, that of CT-measured lung density, are not currently available.
Evidence suggests that there is a correlation between CT-measured lung density and mortality, but
quantifying the importance of the observed effect is not currently possible. As a result, the clinical
benefit of the reported reduction in CT-measured lung density decline is uncertain.
Due to the rarity of the disease, it is challenging to recruit a sample size large enough to adequately
power a study to detect significant differences in the secondary outcomes, e.g. mortality. However,
the
it is not appropriate to attribute the non-significant findings of the secondary outcomes to the lack
of power in the studies.
under Care.
(CTH)
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
72
Page 86 of 218
FOI 5155 - Document 4
Table 42
Balance of clinical benefits and harms of A1PI relative to placebo as measured by the critical patient-relevant outcomes in the key studies
Outcomes (units)
Risk with placebo
Risk with A1PI
Relative effect
Participants
Quality of evidence
Follow-up
(95% CI)
(95% CI)
(studies)
(GRADE)
Comments
Mortality
12 per 1,000
RR 0.35
180
34 per 1,000
⨁⨁⨁⨀
Uncertain due to low event
F/U 24 months
(2 to 78)
(0.05 to 2.27)
(1 RCT)
the MODERATE
rate, RR subject to error
Quality of life (SGRQ)
MD 0.83 points lower
248
-
-
⨁⨁⨀⨀
Direction favours placebo;
F/U 24 to 30 months
(3.49 points lower to 1.82
not statistically significant
points higher)
(2 RCT)
LOW
under
Higher reported RR (1.26,
(CTH) Care.
Annual exacerbation rate
257
-
-
95% CI 0.92 to 1.74), MD
⨁⨁⨁⨀
Direction favours placebo;
F/U 24 to 30 months
(0.36, 95% CI -0.44 to
(2 RCT)
MODERATE
not statistically significant
1.16) in A1PI group
CT-measured lung density
SMD 0.87 g/L higher
304 1982 Aged
-
-
⨁⨁⨁⨁
Direction favours A1PI;
F/U 24 to 30 months
(0.31 higher to 1.42 higher)
(3 RCT)
HIGH
released
statistically significant
Mortality due to treatment-
Act
No reported deaths due to
and
related adverse events
180
No treatment-related deaths were reported
⨁⨁⨁⨀
treatment-related adverse
F/U 24 months
(1 RCT)
MODERATE
events
been
Severe adverse events
283 per 1,000
RR 0.83
257
341 per 1,000
⨁⨁⨁⨁
Direction favours A1PI; not
F/U 24 to 30 months
(195 to 406)
(0.57 to 1.19)
(2 RCT)
HIGH
statistical y significant
has
Health
Discontinuation due to
adverse events
10 per 1,000
RR 0.22
248
of
48 per 1,000
⨁⨁⨁⨀
Direction favours A1PI; not
statistically significant
F/U 24 to 30 months
(2 to 62)
(0.04 to 1.30)
(2 RCT)
MODERATE
Information
Hospitalisation due to
of
adverse events
497
Median rate 1.4% (range 0.0% to 14.3%)
⨁⨁⨀⨀
-
F/U 3 to 6 years
(4 observational studies)
LOW
Abbreviations:
A1PI = alpha-1 proteinase inhibitor,
CI = confidence interval,
CT = computed tomography,
F/U = follow-up,
MD = mean dif erence,
RCT = randomised controlled trial,
RR = relative risk,
SGRQ = St
document
George’s Respiratory Questionnaire,
SMD = standardised mean dif erence.
a GRADE Working Group grades of evidence (Guyatt et al., 2013)
Department
⨁⨁⨁⨁
High quality: We are very confident that the true ef ect lies close to that of the estimate of effect.
This
⨁⨁⨁⨀
Moderate quality: We are moderately confident in the effect estimate: The t
Freedom rue effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially diferent.
⨁⨁⨀⨀
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially dif erent from the estimate of the effect.
the
⨁⨀⨀⨀
Very low quality: We have very lit le confidence in the effect estimate: The true effect is likely to be substantially dif erent from the estimate of effect.
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
73
Page 87 of 218
FOI 5155 - Document 4
SECTION C
TRANSLATION ISSUES
C.1.
OVERVIEW
Three key issues arise in translating the evidence provided in Section B to an economic model
presented in Section D. The first, relates to the applicability of the populations in the pivotal RAPID
trial to clinical practice in Australia; the second, involves selection of utilities; and the third, relates
to the extrapolation of trial evidence beyond the maximum follow-up of the trial. Each issue is
addressed in separate pre-model ing studies in Sections C.2, C.3 and C.4 (Table 43). Each section
provides an overview of the issue to be addressed, the pre-modelling methodology to translate trial
the
data into assumptions for economic modelling, and how results are used in Section D.
Table 43
Outline of Section C issues being addressed
under
Section
Issue
(CTH) Care.
C.2
Applicability of the trial-based evidence to the proposed NBA listing population
C.3
Selection of utilities
1982 Aged
C.4
Extrapolation of trial-based evidence
released
Abbreviations:
NBA = National Blood Authority.
Act and
C.2.
APPLICABILITY TRANSLATION ISSUES
been
C.2.1.
APPLICABILITY OF THE TRIAL-BASED EVIDENCE TO THE PROPOSED MBS POPULATION
has
Health
C.2.1.1
Identification of issue that needs to be addr
of
essed
Information
Applicability relates to any ways in which the participants and circumstances of use in the key RAPID
of
trial presented in Section B, differ from the proposed population for treatment (Chapman et al.
2015). This pre-modelling study addresses whether the definition of the trial population is
document
representative of Australian patients, and whether the circumstances of use of the proposed
medical service in the trial is representative of ho
Department w the service will be used in Australian clinical
This Freedom
practice.
the
C.2.1.2
FOCUSED AN
by
ALYTICAL PLAN
Patient demographic characteristics, along with inclusion and exclusion criteria of included clinical
trials, are reviewed and compared with the proposed NBA-listing eligibility. Inclusion criteria cover
age, and clinical characteristics such as A1PI serum levels ≤11 μM and emphysema defined by FEV1.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
74
Page 88 of 218
FOI 5155 - Document 4
C.2.1.3
RESULTS OF PRE-MODELLING STUDY
Ex or never-smoking individuals with severe A1PI deficiency (serum levels ≤11 μM) and emphysema
(FEV1 <80%) are eligible for AT under eligibility criteria in the PICO Confirmation (DoH, 2016). These
criteria are in line with those currently proposed by the Canadian Thoracic Society (2012)1 and
American Thoracic Society/European Respiratory Society (2003), which recommend that intravenous
AT be commenced for those patients with established airflow obstruction i.e. FEV1 35%-60%
predicted (American Thoracic and European Respiratory Society, 2003; Sandhaus et al. 2016). The
Global Initiative for Chronic Obstructive Lung Disease (GOLD) (2017) suggested that never- or ex-
smokers with an FEV1 of 25%-60% predicted are those most suitable for AT, and that AT should be
provided to those with FEV1 > 65% with a careful analysis of costs.
the
A range of tests is generally performed to confirm eligibility, including A1PI serum levels and
genotype, spirometry, and computed tomography of the lung to assess emphysema. Tests are
undertaken to exclude other conditions. This includes monitoring compliance with smoking
under
(CTH) Care.
cessation, arterial blood gases analysis, sputum examination, and other respiratory function
investigations. The American Thoracic and European Respiratory (2003) indicated that there is
limited evidence to support the use of AT in lung transplant recipients. The demographic and clinical
1982 Aged
characteristics of patients in the RAPID study, RAPID-OLE study, and the UK AATD registry
released
populations are provided in this section. Alignment of patient c
Act haracteris
and tics with the proposed
listing criteria is described.
been
RAPID AND RAPID OLE
has
Health
The RAPID trial (Chapman et al. 2015) is the largest AT tr
of ial undertaken to date. The trial examined
the efficacy and safety of weekly intravenous administratio
Information n (60mg/kg) of Zemaira compared with
placebo over two years in AATD subj
of ects with emphysema (n=180) that were randomised and
treated at 28 trial sites. After the first two years, an open-label extension was undertaken for non-US
document
patients, which is referred to as RAPID-OLE (McElvaney et al. 2017).
Department
The RAPID trial recruited male and female patients, aged 18–65 years, with serum AAT
This Freedom
concentration of ≤11 µM and an FEV1 of 35%–70% of predicted value. Only patients with symptoms
the
of emphysema were included. The average patient age was 53 years, and equal numbers of males
by
and females were recruited. These characteristics are consistent with the proposed listing criteria.
Serum concentration of AAT was below 11 µM and FEV1 % predicted was 47% for both the AT and
BSC arms. Patients who were smokers within six months of recruitment, were lung transplantation
recipients or candidates, had selective IgA deficiency, or were receiving other augmentation
treatments were excluded. The smoking exclusion is in line with the PICO criteria, while lung
1 Non-smoking or ex-smoking patients with COPD (FEV1 25% to 80% predicted) attributable to emphysema and documented A1PI
deficiency (11 mol/L)
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
75
Page 89 of 218
FOI 5155 - Document 4
transplantation exclusion is in line with international guidance. A comparison of the RAPID trial’s
patient population and the proposed listing is presented in Table 44.
Table 44
Comparison of the RAPID trial’s patient population and the proposed listing (Chapman et al. 2015)
Chapman patient baseline characteristics
Patient
Chapman
Characteristic
Zemaira
Placebo
Proposed
characteristics inclusion criteria
N = 93
N = 87
listing
Age
18 to 65 years
Baseline age: mean (SD):
53.8 (6.9)
52.4 (7.8)
18 years or older
Gender
No restriction
Male
52%
57%
No restriction
Baseline CT
TLC
45.5 (15.8)
48.9 (15.5)
lung density
No restriction
FRC
47.6 (15.7)
50.7 (15.0)
No restriction
(g/L)
Combined
46.6 (15.6)
49.8 (15.1)
the
Gender
No restriction
Male (%)
48 (52%)
50 (57%)
No restriction
Ethnicity
No restriction
White (%)
93 (100%)
87 (100%)
No restriction
FEV1 predicted
under
(%)
35–70%
FEV1 predicted (%)
47.4% (12.1)
47.2%
(11.1)
FEV1 <80%.
(CTH) Care.
A1PI serum
concentration
<11μM
A1PI serum concentration
(μM)
(μM)
6.38 (4.62)
5.94 (2.42) ≤11 μM
1982 Aged Ex- or never-
Smoking
No smoking in previous 6
released
months
smoking
individuals
Act and
Waiting list or previous lung
Lung transplant
transplantation, lobectomy,
been
or lung-volume reduction
No restriction
surgery
has
Health
Selective IgA
deficiency
No selective IgA deficiency
No restriction
Information
of
Circumstances of use
Dose regimen: Intraven
of ously 60mg/kg
Regimen
Frequency/duration: Once per week
Not stated
Abbreviations:
A1PI = alpha-1 proteinase inhibitor,
CT = computed tomography,
FEV1 = forced expiratory volume in 1 second,
FRC =
document
functional residual capacity,
IgA = immunoglobulin A,
μM = micromolar,
SD = standard deviation,
TLC = total lung capacity.
The circumstances of use are in line with the proposed listing. RAPID patients were randomised to
This Freedom
Department
receive AT at 60mg/kg every week. The dosing is consistent with the dose approved in the Australian
the
Product Information (PI). The comparator for AT in the RAPID trial was described as best supportive
care, those therapies re
by commended by published treatment guidelines to manage the symptoms
associated with emphysema. BSC encompasses a range of interventions including pharmacological
(e.g. bronchodilators, systemic corticosteroids), non-pharmacological (e.g. oxygen therapy) and
preventative measures such as vaccinations. The optimal mix of therapies varies amongst patients
who are stable and those experiencing acute exacerbation. BSC was provided on the placebo arm of
the RAPID trial, and in addition to AT on the intervention arm.
Other Augmentation Therapy Trials
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
76
Page 90 of 218
FOI 5155 - Document 4
Dirksen et al. (1999) recruited patients from the Danish AAT Deficiency registry from 1991 to 1995
and from the Dutch Registry from 1993 to 1997. The study included 56 patients (26 Danish and 30
Dutch) who were ex-smokers with PI*ZZ genotype-A1PI deficiency and moderate emphysema (FEV1
30%-80% of predicted). AT (PROLASTIN-C, 250mg/kg) was infused every four weeks. This dosing of
62.5mg/kg/week is slightly higher than the listing of 60mg/kg/week. Patients recruited in the study
were in line with the listing (>18 years) and had average ages of 50.4 (Danish) and 45.1 (Dutch)
years, which is similar to the RAPID trial. Average FEV1 % predicted was less than 80%, which aligns
with the proposed listing. The average was 49.4% for Danish subjects and 47.1% for Dutch. This also
compares with the RAPID trial.
Table 45
Comparison of Dirksen and EXACTLE patient population and the proposed listing
the
Dirksen et al. (1999)
EXACTLE
Characteristics and
Danish
Dutch
Proposed
demographics
Subjects
Subjects
PROLASTIN-C Placebo
listing
N = 26
N = 30
N = 38
N = 39
under
(CTH) Care.
Age, years mean (SD)
50.4 (1.62)
45.1 (1.17)
54.7 (8.41)
55.3 (9.80)
>18 years
Sex, male/female %
14/12
20/10
65.8/34.2
41/59.0
None
Smoking pack years mean (SD) 20.0 (2.39)
17.1 (1.90)
Non smoker
1982 Aged
FEV1, mean (SD), % predicted
49.4 (2.75)
47.1 (2.58)
46.33 (19.59)
46.55 (21.05)
FEV1 <80%.
released
FVC % predicted mean (SD)
110 (3.53)
101 (2.92)
Act
None
and
Baseline serum AAT µM
4.62 (1.59)
4.55 (1.68)
≤11 μM
DL
been
CO, mmol/min/kPa %
59.5 (3.28)
61.2 (2.98)
None
predicted mean (SD)
CT, whole lung, g/L mean (SD)
76.7 (6.15)
70.5 (2.14)
Health
None
has
Percentile of lung density [g/L]
46.54 (19.61)
46.84 (17.02)
None
of
Abbreviations:
AAT = alpha-1 antitrypsin,
CT = computed tomography,
DLCO = Pulmonary dif using capacity for carbon monoxide,
FEV1
Information
= forced expired volume in 1 second,
FVC = forced vital capacity,
SD = standard deviation.
Source: Dirksen et al. 1999, Table 1 p 1469. EXACTLE
of CSR Section 11.2 p 64 Table 9 p 65.
The EXACTLE trial was a randomised, double-blind clinical trial that included 77 patients and had a
document
follow-up of two years. Average patient age was 55 years, with a higher proportion of male patients
randomised to AT. Most patients had moderate to severe COPD, based on the GOLD classification
This
Department
system. For inclusion, patients had
Freedom a clinical diagnosis of AATD (serum AAT levels < 11 µM) with a
specific genotype and FEV
the
1 % predicted less than 80% at baseline. These characteristics are in line
with the proposed listing and the RAPID patient population. AT dosing in EXACTLE was consistent
by
with that approved in Australia. A comparison of the Dirksen and EXACTLE patient population and
the proposed listing is presented in Table 45.
UK Anti-trypsin Deficiency Assessment and Program for Treatment (ADAPT), Registry
The UK registry for alpha-1-antitrypsin (AAT) deficiency was established in 1996 and recruitment
started in 1997. Stockley et al. (2015) reported that there were 930 ZZ phenotype and 135 SZ
phenotype highly characterised patients on the database. The author noted that patients have
typically been followed-up on an annual basis, with measurement of health outcomes including
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
77
Page 91 of 218
FOI 5155 - Document 4
health status questionnaires, post-bronchodilator lung-function testing, CT scanning, routine bloods
for haematology and liver function, research bloods for potential biomarkers, whole blood for DNA,
sputum for quantitative culture, and diary cards for monitoring exacerbations. Reported patient
characteristics (Green et al. 2016) are outlined in Table 46. Average patient age of 52.1 years is
similar to the RAPID trial and other previously outlined clinical trials. FEV1 (% predicted) is also in line
with the RAPID trial.
Table 46
Comparison of the UK registry patient population and the proposed listing
Characteristic
All Value
Proposed listing
N= 76
Male patients
44 (57.9)
No restriction
Age (years)
52.1 (14.8)
No restriction
the
Median follow up (years)
7.2 (1.6)
No restriction
FEV1 (% predicted)
45.3 (29.6)
FEV1 <80%.
FEV1/FVC
34.0 (23.0)
No restriction
under
(CTH) Care.
DLCO (% predicted)
64.9 (38.4)
No restriction
KCO (% predicted)
60.5 (28.3)
No restriction
Chronic bronchitis
31 (40.8)
No restriction
1982 Aged
Baseline density (g/l)
46.2 (28.7)
No restriction
released
Change in density/year
-2.13 (4.08)
No restriction
Act and
Density declining
65 (85.8)
No restriction
UZ density
30.33 (26.49)
No restriction
been
LZ density
49.29 (27.58)
No restriction
UZ density decline/year
-1.72 (3.03)
Health
No restriction
has
LZ density decline/year
-1.45 (5.28)
of
No restriction
UZ density declining
54 (77.1)
Information
No restriction
LZ density declining
52 (74.3)
No restriction
of
SGRQ
44.6 (31.2)
No restriction
Abbreviations:
DLCO = Pulmonary dif using capacity for carbon monoxide,
FEV1 = Forced expired volume in 1 second,
FVC = forced vital
document
capacity,
KCO = Transfer factor of carbon monoxide,
LZ = lower zone,
SGRQ = Saint Georges Respiratory Questionnaire,
UZ = upper
zone.
Source: Green et al. 2016, Table 1, p. 83.
This Freedom
Department
C.2.1.4
RELATIONSHIP OF PRE-MODELLING STUDY TO THE ECONOMIC EVALUATION
the
Tonelli and Brantly et al. (2010) indicated that there has been variation in the characteristics of
by
patients selected to receive AT and discordant views on the benefit of such treatment. The
circumstances of use in RAPID, Dirksen, EXACTLE trials and the UK registry are largely consistent with
the target patient population for AT in Australia. The eligibility criteria of FEV1<80% in the draft PICO
corresponds with FEV1 of 35%–70% of the predicted normal value used in the RAPID trial. Dosing
regimens and settings for service delivery used in the RAPID trial are the same as what would be
used in Australian clinical practice. BSC covers a range of lifestyle and pharmacological interventions.
The economic model compares AT plus BSC, with BSC alone, so uncertainty is present on both arms
of the comparison and is likely to have an impact on effectiveness. Mortality and morbidity severity
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
78
Page 92 of 218
FOI 5155 - Document 4
(proportions hospitalised by COPD state) are subject to sensitivity analysis to gauge how this
uncertainty could affect the estimated ICER.
C.3.
SELECTION OF UTILITY VALUE ISSUES
C.3.1
IDENTIFICATION OF ISSUE THAT NEEDS TO BE ADDRESSED
A1PI deficiency impacts patient quality of life. For example, Dirksen et al. (2009) reported that the
QoL of patients at baseline in the EXACTLE study was significantly impaired, as measured by the St
Georges’ Respiratory Questionnaire (SGRQ). Generic measures of QoL, such as EuroQol Group 5
domain questionnaire (EQ-5D), were not reported in the RAPID trial as the trial was powered to
measure treatment effect on changes in CT-scan lung density and pulmonary function tests. Larger
the
numbers of patients would be required to measure outcomes using this approach. Cost-utility
analysis requires the derivation of quality of life outcomes, as measured by instruments such as EQ-
5D, or other generic questionnaires.
under
(CTH) Care.
C.3.2
FOCUSED ANALYTICAL PLAN
The literature was reviewed to determine EQ-5D values for AATD patients suffe
Aged ring COPD of
1982
differing severity. Specifically, values were sought where COPD h
released ad been stratified by FEV1%
Act
predicted. QoL data is derived from these sources for inclusion as utility
and values in the economic
model.
been
C.3.3
RESULTS OF PRE-MODELLING STUDY
has
Health
of
Literature search for FEV1% Predicted Health States
Information
There is limited published data on AATD survival and quality of life because of the rare nature of the
of
disease. A literature search was conducted in EMBASE, Cochrane Library, and HTA agency websites
including CADTH and NICE on 20 Ju
document ne 2018 to identify published quality of life analyses for AATD
patients. The search strategy involved the search terms included in Table 47. Titles and abstracts
Department
were reviewed and a manual search was performed.
This Freedom
the
Table 47
Search strategy for AATD utility literature review
by
Search
Terms
1
[AATD] OR [alpha-1 antitrypsin deficiency] OR [antitrypsin deficiency]
2
[AQoL] OR [Australian quality of life] OR QALY
3
[EQ-5D] OR [SGRQ] or [HRQL]
4
[SF-6D] OR [short form 6D]
5
[Time trade off] OR [TTO] OR [Standard gamble]
6
[Health utilities] OR [utility values] OR [utility scores]
7
[2] OR [3] OR [4] OR [5] OR [6] OR [7]
8
[1] AND [8]
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
79
Page 93 of 218
FOI 5155 - Document 4
Table 48
Results of AATD utility literature review
EMBASE
Other HTA
Cochrane
websites a
Library
Number of titles and abstracts reviewed after search
53
1
TOTAL number of exclusions
49
0
Number of AATD utility studies included
4
1
Consolidated number of studies excluding duplicates
5
a HTA agencies included: NICE, CADTH.
As expected, there were a limited number of publications reporting on studies assessing the impact
of AATD on patient QoL. Five relevant publications were identified (Table 48). A number of reviews
of COPD economic models have recently been conducted (Table 49). These models also assess QoL
in relation to FEV
the
1 severity. This is relevant to AATD, even though AATD is related to severe
emphysema, rather than a broader range of conditions under the COPD classification of lung
disease. Two of these recent COPD utility reviews are also included in this section as background.
under
(CTH) Care.
Table 49
Studies identified outlining utilities for AATD and COPD states
Study
Reference
Utilities for AATD
1982 Aged
Ejiofor and
Ejiofor & Stockley, Health status measurements in AATD. European Respiratory Journal 2015 46: PA1032;
released
Stockley
DOI: 10.1183/13993003.congress-2015.PA1032
Act and
2015
Manca et al.
Manca S, Rodriguez E, Huerta A, Torres M, Lazaro L, Curi S, Pirina P, Miravitl es M. Usefulness of the
2014
CAT, LCOPD, EQ-5D and COPDSS scales in under
been standing the impact of lung disease in patients with
alpha-1 antitrypsin deficiency. COPD. 2014 Sep;11(5):480-8. doi: 10.3109/15412555.2014.898030.
Gøtzsche and Gøtzsche and Johansen 2016 Intravenous alpha-1 antitrypsin augmentation therapy for treating patients
has
Health
Johansen
with alpha-1 antitrypsin deficiency and lung disease2, Cochrane Database of Systematic Reviews
2016
of
Bernhard et
Bernhard N.; Lepper P.M.; Vogelmeier C.; Seibert M.; W
Information agenpfeil S.; Bals R.; Fahndrich S. Deterioration of
al. 2017
quality of life is associated with the exacerbation frequency in individuals with alpha-1-antitrypsin deficiency
of
- Analysis from the German registry. International Journal of COPD. 12 (pp 1427-1437), 2017. Date of
Publication: 12 May 2017.
Carone et al. Carone M.; Bruletti G.; Bertella E.; Balestroni G.; Gatta N.; Corda L.; Luisetti M.; Balbi B. Quality of life
document
2011
evaluation in patients with alpha-1-anti-trypsin deficiency: A 3-year prospective study. European
Respiratory Journal. Conference: European Respiratory Society Annual Congress 2011. Amsterdam
Netherlands. Conference Publication: (var.pagi
Department ngs). 38 (SUPPL. 55) (no pagination), 2011. Date of
This
Publication: 01 Sep 2011.
Freedom
Utilities for COPD models – recent reviews
the
Moayeri et al. Moayeri F, Hsueh YS, Clarke P, Hua X, Dunt D. Health State Utility Value in Chronic Obstructive
2016
Pulmonary D
by isease (COPD); The Challenge of Heterogeneity: A Systematic Review and Meta-Analysis.
COPD. 2016 Jun;13(3):380-98. doi: 10.3109/15412555.2015.1092953.
Hoogendoorn Hoogendoorn M, Feenstra TL, Asukai Y, Briggs AH, Borg S, Dal Negro RW, Hansen RN, Jansson SA,
et al. 2016.
Leidl R, Risebrough N, Samyshkin Y, Wacker ME, Rutten-van Mölken MPMH Patient Heterogeneity in
Health Economic Decision Models for Chronic Obstructive Pulmonary Disease: Are Current Models
Suitable to Evaluate Personalized Medicine? Value Health. 2016 Sep - Oct;19(6):800-810. doi:
10.1016/j.jval.2016.04.002
Abbreviations:
AATD = alpha-1 anti-trypsin deficiency,
COPD = chronic obstructive pulmonary disease.
2 http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD007851.pub3/ful
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
80
Page 94 of 218
FOI 5155 - Document 4
Utilities for AATD
Ejiofor and Stockley 2015
CSL Behring presented the health-related QoL data prepared by Ejiofor and Stockley (2015) for 244
patients not receiving AT in the UK ADAPT programme in 2014. The data records post-
bronchodilator FEV1 and the EQ-5D utility values. Results are presented in Table 50, with the EQ-5D
ranging from 0.51 for those with FEV1<30% predicted, to 0.79 for FEV1>50%. Limited information is
provided about how EQ-5D values were derived, although it is evident that patients with FEV1>50
comprised 65% of the patient population.
s45, s47(1)(b)
the
under
(CTH) Care.
Aged
released 1982
Act and
been
Data plots were also provided in the Ejiofor and Stockley (2015) study, where the relationships
Health
between EQ-5D, SGRQ and FEV
has
1 predicted values were examined. EQ-5D data correlated with the
of
results for the SGRQ (R=-0.772 p<0.001). FEV1 was considered to explain approximately 43% of the
Information
variation in health status as assessed by these instruments, thus factors other than FEV1 also have an
of
important impact on health status (Ejiofor and Stockley, 2015).
document
SGRQ was also measured in the RAPID trial. The small sample size appears to confound any possible
changes in QoL as measured by this instrument. The authors concluded that, “unsurprisingly,
Department
findings from our s
This tudy did not show significant differences between active and placebo treatment
Freedom
in conventional pulmonary fu
the nction and clinical endpoints; the study was not designed with
sufficient power to detect such changes” (Chapman et al. 2015 p. 366). This lack of significance limits
by
the QoL assessment measured in the RAPID trial being related back to EQ-5D-SGRQ correlations
observed by Ejiofor and Stockley (2015).
Manca et al. 2014
The authors aimed to assess the usefulness of different instruments to evaluate QoL in COPD
patients with and without AATD. A total of 96 patients were included, 35 with AATD (average age
56.5 years and mean FEV1% 48.7%) and 61 with non-AATD COPD (70.3 years and FEV1% 47%). All
patients completed the COPD severity score (COPDSS), the EQ-5D, the Living with COPD (LCOPD) and
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
81
Page 95 of 218
FOI 5155 - Document 4
the COPD Assessment Test (CAT) questionnaires. Questionnaire scores were similar for non-AATD
COPD and AATD patients. For example, the average EQ-5D index score was 0.74 for AATD and 0.72
for non-AATD COPD patients. In general, the correlations of scores with FEV1(%) were higher for
AATD patients compared with non-AATD COPD patients. Those with AATD were usual y younger,
with fewer co-morbidities, and less likely to be smokers.
Bernhard et al. 2017
The aim of this study was to provide information about the deterioration in QoL over a maximum
follow-up period of seven years (median follow-up 3.33 years) in AATD patients. Data from the
German AATD registry was mined in relation to SGRQ score, exacerbation frequency, smoking
history, FEV1 and DLCO across 868 individuals with PiZZ genotypes. Average patient age was 52.6
the
years and average SGRQ score was 45.7. SGRQ was correlated with exacerbation frequency, FEV1,
smoking and age. Mean annual decrease of SGRQ score in 286 followed-up patients was 1.21 points
per year. Worsening of SGRQ was associated with exacerbation frequency in i
under ndividuals with PiZZ
(CTH) Care.
AATD
Gøtzsche and Johansen 2016
Aged
released 1982
These authors reviewed the benefits and harms of AT using the Cochrane Central Register of
Act and
Controlled Trials, PubMed and ClinicalTrials.gov to March 2016. Data for QoL was limited, with the
authors only identifying two trials that reported
been QoL using the SGRQ. The annual rate of
exacerbations could not be included in meta-analysis, as the distribution of the values was highly
has
Health
skewed.
Information
of
Carone et al. 2011
of
The aim of this study was to evaluate QoL in relation to AT use (25 patients) and non-AT (7 patients)
as part of a three-year prospective
document analysis of 32 patients (average age 54 years and FEV1 48%
predicted). The SGRQ and EQ-5D questionnaires were administered at baseline and yearly for three
Department
years. After three y
This ears, the decrease in FEV1 in the AT group was 125 ml (4%), whereas in the non-
Freedom
AT group the decrease was 610 ml (41%), (p<0.02). SGRQ changes were significantly different. The
the
AT arm showed a 7.8-unit improvement, whereas in the non-AT arm QoL decreased by 7.9 units.
by
Changes in health status between the two groups were not significant using EQ-5D.
Utilities for COPD models
Only a limited number of AATD studies were identified that reported quality of life. Given the small
number of AATD-specific studies, some recent reviews of utilities employed in COPD economic
models are also included in this section as further background.
Moayeri et al. 2016
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
82
Page 96 of 218
FOI 5155 - Document 4
Moayeri and col eagues undertook a systematic review3 to estimate mean utility values for COPD
using meta-analysis and explored the degree of heterogeneity in the utility values across a variety of
clinical studies. Simulation-based studies were not included. The authors limited their analysis to
studies using EQ-5D to estimate utility values because it is the most widely used generic measure
across all diseases and includes dimensions of mobility, self-care, usual activities, pain and anxiety,
which are converted to a single index using preference weights. Country-specific algorithms or tariffs
have been generated (Dolan, 1997, Tsuchiya et al. 2002) for this weighting and a minimally
important clinical difference for the EQ-5D Index has been estimated to be 0.074 (Walters & Brazier
2005).
The authors identified 32 COPD studies using EQ-5D with 49 observations. Seventeen studies
the
reported utility values by severity of COPD stage. Utility values are outlined in Table 51 (Taken from
Table 3, p. 388) for studies were utilities were reported for 3, or more states. They ranged from 0.91
for stage I to 0.41 for stage IV. GOLD stage 1 (very mild COPD with FEV1>80% predicted) utility
under
values ranged from 0.73 to 0.91, while GOLD stage 4 (severe emphysema with FEV1 <
Care. 30 %
(CTH)
predicted) utilities ranged from 0.52 to 0.78. The average values for COPD Stages 1-2 (0.78) and
Stages 3-4 (0.67) are similar to that for AATD-specific COPD reported by Ejiofor and Stockley, (2015)
1982 Aged
for Stages 1-2 of 0.79 and more than 0.59 for Stages 3-4. Results of the Fourth and Fifth Korea
released
National Health and Nutrition Examination Survey reported by Kim et al. (2014) skew COPD results
Act and
for Stages 3-4. Average utilities are similar for most stages despite COPD relating to more conditions
than emphysema. Manca et al. 2014 reported QoL m
been easurement instrument4 scores for AATD and
non-AATD COPD patients to be similar.
has
Health
Table 51
Selected EQ-5D values stratified by GOLD (FEV
of
1%) states from Moayeri et al. 2016
Information
Study
Staging Scores
Method
of
I 0.786
This 2011 study included a cross-sectional survey of 678 COPD patients in China
Wu et al.
GOLD
II 0.734
using the EQ-5D questionnaire. The authors found that age, gender and disease
2015
Stage
III 0.691
severity were significantly associated with quality of life after taking other covariates
document
IV 0.655
into consideration.
I 0.83
This
Department
Kim et al. GOLD
II 0.88
The E
Freedom Q-5D and Clinical COPD questionnaires were completed by 200 Korean
2014
Stage
III 0.81
patients with COPD in one tertiary hospital.
the
IV 0.60
by
I 0.906
The Fourth and Fifth Korea National Health and Nutrition Examination Survey was
Kim et al. GOLD
II 0.912
used which included 20,261 adults above 40 years. Mean utility of COPD patients
20145
Stage
III 0.857
was 0.906(SE 0.004) compared to 0.922(SE 0.001) in the non-COPD control
IV 0.780
group.
3 MEDLINE, EMBASE, Web of Science, CINAHL, ProQuest, Cochrane Library, Health Technology Assessment Database, International Society
for Pharmacoeconomics and Outcomes Research (ISPOR) and Google Scholar
4 COPD severity score (COPDSS), the EuroQoL 5-Dimensions (EQ-5D), the Living with COPD (LCOPD) and the COPD Assessment Test (CAT)
questionnaire
5 Kim ES, Lee BJ, Lee GW, Jung AR, Hwang HS. Health status in adult patients with COPD in Korea. Value Health 2014; 17(7):A779–A780
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
83
Page 97 of 218
FOI 5155 - Document 4
Study
Staging Scores
Method
I 0.82
EQ-5D questionnaires were implemented in three indacaterol phase II clinical trials
Asukai et GOLD
II 0.801
at baseline, and weeks 12 and 26 (end of the studies). The EQ-5D questionnaire
al. 2012
Stage
III 0.774
was completed at the same time as pre-bronchodilator FEV1 assessment. Around
IV 0.743
11,000 EQ-5D questionnaires were pooled and analysed.
Mild 0.84;
Fletcheret
Moderate
2426 participants aged 45-67 were recruited to a multi-country study (Brazil, China,
al. 2011
BTS
0.58;
Germany, Turkey, US, UK) and cross-sectional survey undertaken. Two thirds of
Severe 0.41 patients had either moderate or severe COPD.
I 0.73
Pickard et GOLD
II 0.59
120 hospitalised COPD patients self-completed EQ-5D and SF-36 surveys and the
al. 2011
Stage
III 0.63
disease-specific SGRQ. EQ-5D were transformed using UK tarif s.
IV 0.63
II 0.752
The study collected QoL data as part of the TORCH (Towards a Revolution in
the
Starkie et GOLD
COPD Health) trial. SGRQ and EQ-5D surveys were implemented at baseline and
al. 2011
Stage
III 0.708
every 24 weeks for 3 years. The study included 6112 participants (4236 completed
IV 0.672
EQ-5D surveys).
I 0.68-0.77
under
Punekar
(CTH) Care.
et al.
GOLD
II 0.68-0.72 This cross sectional multi-country (five EU countries and USA) survey included
2007
Stage
III 0.62-0.64 2703 patients and their clinicians (1381 in primary and 1322 in specialty care).
IV 0.655
1982 Aged
Rutten
GOLD
QoL was measured using EQ-5D visual analogue scale (VAS) scores, EQ-5D utility
released
van
Stage
II 0.787
scores, and SGRQ from patients in a 4-year Tiotropium trial. 1,235 patients
Moleken
Act
UK
III 0.75
participated from 13 countries. The authors noted EQ-5D VAS and utility scores
and
et al.
dif ered significantly among patients in GOLD stages 2, 3, and 4, also after
value
IV 0.647
2006
correction for age, sex, smoking, body mass index (BMI), and comorbidity (p <
set
0.001).
been
I 0.84
Health
Stahl et
GOLD
II 0.73
174 COPD patients from Sweden self-completed Short Form 36 (SF-36), SGRQ,
has
al. 2003
Stage
EQ-5D, Health States-COPD (HS-COPD), and Work Productivity and Activity
III 0.74
of
Impairment Questionnaire for COPD (WPAI-COPD) questionnaires.
IV 0.52
Information
I 0.8971
of
Borg et
GOLD
I 0.7551
The study used a cost-of-il ness study in northern Sweden and expert derived from
al. (2005) Stage
III 0.7481
a study of asthma in the UK.
document
IV 0.5493
Abbreviations:
BMI = body mass index,
COPD = Chronic Obstructive Pulmonary Disease,
EQ-5D = euroqol group 5 domain
questionnaire,
FEV1 = Forced expired volume in 1 second,
GOLD = global initiative for chronic obstructive lung disease,
QoL = quality of
Department
life,
SGRQ = Saint Georges
This Respiratory Questionnaire,
VAS = visual analogue scale.
Freedom
Many of the identified COPD
the simulation models described in the economic model background
section of this report include utility values from a number of key trials. A selection of these models is
by
summarised in Table 52. Many of the economic models were developed as part of evaluations
associated with the trial. Most models estimate utility by FEV1-defined COPD states under stable
disease and also during exacerbation. For example, in the Asukai Markov model, the indacaterol
phase II clinical trial program collected EQ-5D data during selected patient visits.6This data was
mapped back to FEV1 mild to severe categories, with mild being assigned 0.82, moderate 0.80,
6 Whenever an EQ-5D questionnaire was completed at a time for which a pre-bronchodilator FEV1 value was available
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
84
Page 98 of 218
FOI 5155 - Document 4
severe 0.77 and very severe 0.74. No utility data were available from the trials to describe an
exacerbation and therefore these values were based on the literature.
Table 52
Selected utility values from COPD models outlined by Hoogendoorn et al. 2017
Study
Utilities during Values
Utilities during
Values
Study Design, age
Stable disease
exacerbations
specified by
specified by
EQ-5D was completed at a
time for which a pre-
bronchodilator FEV1 value
Non-severe
Asukai
Mild 0.82
was available, EQ-5D score
exacerbation
Markov
Moderate 0.80
Exacerbation
-0.01
was labelled as describing
Price et al. FEV1% pred
Severe 0.77
severity
the corresponding disease
the
2011
Severe exacerbation
Very severe 0.74
-0.08
severity. No utility data were
available from the trials to
describe an exacerbation
under
(CTH) Care.
QALY weights at
COPD I 0.8971
exacerbations were
Exacerbation
Mild U x 0.95
Borg et al.
COPD IIA 0.7551
expressed as
Aged a fraction of
1982
2004
FEV1% pred
COPD IIB 0.7481 severity, FEV1%
Moderate U x 0.85 the exacerbation-free weight
released
COPD III 0.5493 pred*
Severe U x 0.3
Act
by COPD severity
and
EQ-5D utility weights were
been
specified by COPD severity
Health
from Borg et al. 2004.
has
Mild 0.8971
O’Reilley et al. presented
of
Hoogendoorn
Moderate 0.7551 Exacerbation
utility values at admission
Information
et al. 2011 FEV1% pred
Severe 0.7481
Very Severe
severity
n/a
and discharge for a COPD
of
0.5493
hospitalization based on the
UK for severe exaberations
document
and Goosens for moderate.
Department
-0.12 for 1 month
This Freedom
(moderate
Selected subgroup analyses
Sever
the e COPD
exacerbation of 0.01) for patients with at least two
Samyshkin et
0.751
Exacerbation
and 0.504
al. 2014
FEV1% pred
COPD exacerbations in the
by Very severe severity
(representing a loss
0.657
of QALY per severe previous year.
exacerbation of
0.042)
Abbreviations:
COPD = Chronic Obstructive Pulmonary Disease,
EQ-5D = euroqol group 5 domain questionnaire,
FEV1 = Forced
expired volume in 1 second, *
pred = predicted
QALY = quality-adjusted life year,
UK = United Kingdom.
The Borg et al. (2004) model took a similar approach. Results of a EQ-5D quality-of-life questionnaire
from a cost-of-illness study were used for quality-adjusted life-years (QALY) weights. Hoogendoorn
et al. (2011) used the same approach, with the annual number of QALYs being calculated as the
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
85
Page 99 of 218
FOI 5155 - Document 4
annual number of life years using Q-5D utility weights specified by COPD severity (Borg et al. 2004).
For each exacerbation a decrement in utility weights was applied.
Samyshkin et al. (2014) also employed health-related utilities for stable disease and exacerbations in
their model. The severe COPD and very severe COPD states were associated with utility values of
0.751 and 0.657, respectively. Hospital-treated exacerbations and community-treated exacerbations
were translated into loss of QALY per an event of exacerbation. Hoogendoorn et al. (2017) noted
COPD models employ differing utility values to similar COPD stages and utility decrements assigned
to exacerbations. For instance, the reported average utility values for stage II COPD range from
0.579 (Fletcher et al. 2011) to 0.929 (Rutten et al. 2009). Different methods of utility elicitation
measures were thought to explain this variability.
the
Stage 1-2 and 3-4 COPD stage utility values appear to be broadly aligned in many of the AATD and
COPD studies listed in this review. Hesselink et al. 2006 reported that changes in FEV1% predicted
weakly correlated with utility changes during a two-year follow-up of COPD pa
under tients, implying that
(CTH) Care.
clinical measures such as FEV1% predicted provide limited information about health condition and
are not well correlated with health status of COPD patients. The updated 2014 GOLD report suggests
that progression and severity is best measured by a combined COP
1982 D assessm
Aged ent, including
spirometric test, risk of exacerbations and COPD Assessment
released Test (CAT) or COPD Control
Act and
Questionnaire (CCQ).
been
The SGRQ is a QoL measurement tool that captures three health domains of symptoms, activity and
impact on daily life. As part of the TORCH (Towards a Revolution in COPD Health) trial the SGRQ and
has
Health
EQ-5D were measured every 24 weeks for three years. Around 18,505 observations included EQ-5D
of
index and SGRQ scores. A simple algorithm was develope
Information d to transform SGRQ into EQ-5D values.
The SGRQ was administered as part of the RAPID trial, however, no differences were found in SGRQ
of
between treatment arms possibly due to limited patient numbers. Estimation of EQ-5D differences
from SGRQ results in RAPID is therefo
document re not possible, however, difference in utilities between AT and
BSC arms are unlikely to be substantial, given RAPID’s SGRQ findings.
This Freedom
Department
Utilities for Lung Transplantation Health State
the
A literature search to identify published QoL analyses associated with lung transplant was conducted
by
on 20 June 2018 in EMBASE, Cochrane Library, and HTA agency websites including CADTH and and
NICE. The search strategy involved the search terms included in Table 47, except that AATD was
substituted by lung transplant and lung transplantation. Titles and abstracts were reviewed and a
manual search was performed. A total of 59 titles were identified, with six being deemed as relevant
(see Table 53).
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
86
Page 100 of 218
FOI 5155 - Document 4
Table 53
Utilities for lung transplantation
Study
Reference
Utilities for lung transplant
TenVergert et al.
TenVergert EM, Essink-Bot ML, Geertsma A, van Enckevort PJ, de Boer WJ, van der BW. The effect
1998
of lung transplantation on health-related quality of life: a longitudinal study. Chest 1998; 113: 358–364
van Den Berg et
van Den Berg JW, Geertsma A, van Der BIJ, et al. Bronchiolitis obliterans syndrome after lung
al. 2000
transplantation and health-related quality of life. Am J Respir Crit Care Med 2000; 161: 1937–1941
Groen et al. 2004 AC, McGuire, A, Rogers, CA & Murday, AJ, 2004. Cost-Effectiveness of Lung Transplantation in
Relation to Type of End-Stage Pulmonary Disease. American Journal of Transplantation, 4(7), pp.
1155-62.
Anyanwu et al.
Anyanwu, AC, McGuire, A, Rogers, CA & Murday, AJ, 2001. Assessment of quality of life in lung
2001
transplantation using a simple generic tool. Thorax, Volume 56, pp. 218-22.
Singer et al. 2009 Singer L.G.; Chowdhury N.; Chaparro C.; Hutcheon M.A. 2009. Post-lung transplant health-related
the
quality of life: Perception and reality, Journal of Heart and Lung Transplantation. Conference: 29th
Annual Meeting and Scientific Sessions of the International Society for Heart and Lung
Transplantation. Paris France. Conference Publication: (var.pagings). 28 (2 SUPPL. 1) (pp S127),
2009
under
Singer et al. 2015 Singer L.G.; Chowdhury N.A.; Faughnan M.E.; Granton J.; Keshavjee S.; Marras T.K.; Tullis D.
Care.E.;
(CTH)
Waddell T.K.; Tomlinson G.2015, Ef ects of recipient age and diagnosis on health-related quality-of-life
benefit of lung transplantation, American Journal of Respiratory and Critical Care Medicine. 192 (8) (pp
965-973), 2015.
1982 Aged
TenVergert et al. 1998
released
Act and
The aim of this study was to assess the change in health-related quality of life (HRQL) among 24
been
Dutch lung transplant patients before and after transplantation to treat emphysema. Patients self-
completed questionnaires7 before transplantation, and at 1, 4, 7, 13, and 19 months after
has
Health
transplantation. Transplantation improved mobility, energy and depression. This benefit was
of
maintained for 19 months post-transplantation. Bronchiolitis obliterans (BOS) was highlighted as the
Information
most frequent cause of late morbidity in lung transplant recipients. Of the 24 patients included in
of
the present study, six patients developed BOS within 19 months.
document
Singer et al. 2009
Department
These authors calc
This ulated the difference between mean pre-transplant and post-transplant utilities
Freedom
across 252 patients, for both
the Standard Gamble (SG) and Visual Analogue Scores (VAS). Utility
improved with transplant, even for those with BOS. The mean SG utility pre-transplant was 0.4,
by
which increased to 0.88 without BOS and 0.75 with BOS. VAS pre-transplant was 36 and increased to
77 without BOS and 63 with BOS. The authors concluded that lung transplantation significantly
improves utility, even with BOS.
van Den Berg et al. 2000
7 Nottingham health profile (NHP), the State-trait Anxiety Inventory, the Self-Rating Depression Scale-Zung, the Karnofsky Performance
index, the index of wel -being, and activities of daily living (ADL)
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
87
Page 101 of 218
FOI 5155 - Document 4
The aim of the study was to assess the relationship between health-related QoL and BOS in the
Groningen Lung Transplant Program. The study involved cross-sectional comparison of those with
and without BOS, and a longitudinal analysis of 22 patients. It that found lung transplant patients
with BOS had lower QoL, which persisted for two to three years post-transplantation.
Singer et al. 2015
QoL was assessed on 430 patients using the SGRQ, EQ-5D, SG, VAS and 36-Item Short-Form Health
Survey (SF-36). Transplantation conferred large improvements across all instruments. The SGRQ
decreased 247 units, EQ-5D improved by 0.27, SG by 0.48, and VAS by 44. Age was not associated
with significant differences in QoL benefits.
the
Groen et al. 2004
QoL data was sourced from the Dutch lung transplantation program between 1991 and 1995 using
under
EuroQol questionnaires taken every three months for patients on the transplant waiting list,
Care. along
(CTH)
with one, four and seven months post-transplantation, and then every six months. For those on the
waiting list, average utility values of 0.55 during the first six months, 0.50 between six and nine
1982 Aged
months, 0.45 between nine and 12 months, and 0.40 after one year. Post-transplantation mean
released
utility values ranged from 0.69 at one month post-transplantation to 0.83-0.85 at three-12 months
Act and
after transplantation. Utility increased to 0.91 in the following year.
been
Anwanyu et al. 2001
has
Health
The authors calculated utility scores in a cross-sectional sample of 87 patients waiting for
of
transplantation and 255 lung transplant recipients in the UK
Information . Mean patient age was 39 years, with 28
single lung transplants, 24 bilateral and
of 34 heart-lung transplants. Utility was measured using a self-
completed EQ-5D questionnaire. The mean utility value of patients on the waiting list was 0.31.
Average utility values for recipients
document three years post-transplantation were 0.61 for single, 0.82 for
bilateral, and 0.87 for heart-lung transplants. These values are higher than at 0-6-months post-
Department
transplant, where 0
This .69 for single, 0.75 for bilateral, and 0.67 for heart-lung transplants were
Freedom
reported.
the
by
C.3. 4. RELATIONSHIP OF PRE-MODELLING STUDY TO THE ECONOMIC EVALUATION
Utilities for FEV1 Health States
Both AATD and non-AATD COPD patients have taken part in studies where QoL has been elicited
using a range of methods including SGRQ, SF-36, EQ-5D, SG, and VAS. Utility data is only available by
FEV1% predicted values from the UK registry for AATD patients provided in the Ejiofor and Stockley
(2015) study. QoL assessment is problematic as there was weak FEV1 and EQ-5D-SGRQ correlation
observed by Ejiofor and Stockley (2015). The COPD literature also noted that FEV1 was a relatively
poor indicator of QoL, and composite instruments such as CAT or SQRQ tools should be combined
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
88
Page 102 of 218
FOI 5155 - Document 4
with spirometry to assess QoL. The RAPID trial implemented SQRQ, however, no significant
differences between treatment arms were found.
Given the paucity of available data and limited accuracy of mapping EQ-5D by FEV1 predicted, utility
values are assigned for patients with FEV1 above and below 50% predicted. GOLD Stages 1-2 EQ-5D
utilities averaged around 0.7-0.9 among the reviewed COPD studies and 0.5-0.8 for GOLD Stages 3-4.
Similar averages were evident in Ejiofor and Stockley (2015) for COPD patients with AATD and are
used in the economic mode. Table 54 presents the health state utility values applied in Section D
cost-effectiveness model.
The Ejiofor and Stockley (2015) average of 0.79 for FEV>50% predicted and 0.59 for patients with
FEV<50% are used. This approach does not attempt to include disutility associated with exacerbation
the
frequency. Given this uncertainty, sensitivity analysis is undertaken in the concluding part of Section
D to understand the sensitivity of ICER results to changes in utility/FEV1 assumptions. It is evident
that model results are relatively robust, and that much of the economic b
under enefit is driven by
(CTH) Care.
increases in life expectancy associated with AT.
Table 54
Summary of utility inputs for the Section D cost-effectiveness mode
1982 Aged
Health state
Utility
Nature of
Source
Alternative
Source
released
estimate
estimates of
Act and
utility value
been
FEV1 ≥50% predicted (all
0.79
EQ-5D data
Mean utility
+/- 5%
Sensitivity
rates of lung density decline)
score based on
analysis
UK Registry
has
Health
data (Ejiofor
of
FEV1 <50% predicted (all
0.59
EQ-5D data
Mean utility
+/- 5%
Sensitivity
Information
rates of lung density decline)
score based on
analysis
UK Registry
of
data (Ejiofor
First year of lung transplant
0.74
EuroQoL data
Mean EuroQoL +/- 5%
Sensitivity
document
score (mos 0-
analysis
18), Anwanyu
2001
This
Department
Subsequent years following
0.77
Freedom
EuroQoL data
Mean EuroQoL +/- 5%
Sensitivity
lung transplant
score (mos 19-
analysis
the
36+ months),
Anwanyu 2001
by
Abbreviations:
EQ-5D = euroqol group 5 domain questionnaire,
FEV1 = Forced expired volume in 1 second,
NR = not reported.
Utilities for Lung transplant
Lung transplant utility results reported by Groen et al. (2004) and Anyanwu et al. (2001) indicate that
utility increases substantially after transplantation. The data from Anyanwu et al. (2001) are used in
the base case (provided in Table 54), as limited detail is provided about the small sample of patients
used in the Groen et al. (2004) study. Average utilities for single, double and heart-lung (from Table
2, p. 413) of 0.74 for 0-18 months and 0.77 for 19-36 months+ are included. Utility values were
shown to be age insensitive by Singer et al. (2015). The incidence of BOS appeared to be a key driver
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
89
Page 103 of 218
FOI 5155 - Document 4
of estimated utility. The values are subject to sensitivity analysis to ascertain the robustness of
results to transplant QoL assumptions.
C.4.
EXTRAPOLATION TRANSLATION ISSUES
C.4.1
IDENTIFICATION OF ISSUE THAT NEEDS TO BE ADDRESSED
This pre-modelling study addresses the method for extrapolating data from the four-year RAPID trial
period as an input into the lifetime economic evaluation. This includes transition probabilities
between FEV1 and CT-scan lung density states, and survival.
C.4.2
FOCUSED ANALYTICAL PLAN
the
Patient transition between health states in the RAPID trial are used for the first four years of the
model timeframe for AT and for two years for BSC, as the RAPID-OLE extension was confined to the
AT arm. Discussions with clinical experts indicate that AATD patients stabilise a
under fter four years. It is
(CTH) Care.
assumed that after the first four years of the modelling timeframe, patients will remain on either no
decline, slow or rapid decline CT-scan lung density pathways over the 26-year lifetime projection.
Parametric survival models are fitted to UK registry data to project survival for p
Aged atients in each
1982
health state from Year five over the lifetime projection.
released
Act and
C.4.3
RESULTS OF PRE-MODELLING STUDY
been
Transition between FEV1 and lung density decline states
has
Health
s45, s47(1)(b)
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
90
Page 104 of 218
FOI 5155 - Document 4
s45, s47(1)(b)
The average decline in FEV1% predicted
for 406 A1PI deficiency patients was 1.45% per year in the UK registry (Stockley 2015).
s45, s47(1)(b)
the
under
(CTH) Care.
1982 Aged
A slower rate of progression is possible for patients using AT. s45, s47(1)(b)
released
Act and
been
has
Health
Information
of
of
Survival
Survival estimates in the RAPID trial
document are the primary clinical inputs in the economic evaluation and
the basis from which extrapolation occurs. Annual mortalities on the BSC and AT arms of the RAPID
Department
and RAPID-OLE stu
This dies were used for the first two and four years of the model, respectively.
Freedom
Extrapolation of survival after two and four years for the BSC and AT arms was required to estimate
the
the proportion of patients in each health state across the time horizon of the model.
by
s45, s47(1)(b)
. A number of studies have shown FEV1 to be an
important predictor of survival in patients with emphysema. Two-year mortality increases
exponential y once FEV1 falls below one-third of predicted, at which point two-year mortality
reaches 50% in patients with FEV1 of 15% of predicted (Seersholm et al. 1994). Annual probabilities
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
91
Page 105 of 218
FOI 5155 - Document 4
of survival derived from parametric model extrapolations were assumed to be the same for each
health state regardless of treatment arm.
FEV1 >50 survival
Green et al. (2016) presented AATD patient subgroup survival analyses based on FEV1 which
“showed an apparent separation of curves on Kaplan Meier plots suggesting that there may be an
effect on mortality in the patients with a presentation FEV1>30% predicted. The analysis was not
statistically significant presumably due to inadequate power- as the number of deaths per group was
low” (ibid, p. 86). s45, s47(1)(b)
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
92
Page 106 of 218
FOI 5155 - Document 4
Table 55
Goodness of fit and parameters for FEV 1 >50 survival models
Parametric model
AIC
Shape
Scale
Other
(meanlog) (mu)
(sdlog) (sigma)
(Q)
Weibull
88.76
2.5700
15.5650
Exponential
93.68
0.02776
Lognormal
90.89
2.865
0.848
Generalised gamma
90.21
2.73E+00
1.56E-01
2.61E+00
Gompertz
86.84
3.98E-01
2.69E-03
Log-logistic
89.30
2.681
14.652
Abbreviations:
AIC = Akaike’s information criteria.
FEV1 <50 no decline
the
The Weibull survival function corresponds to a mortality rate that increases with time. The
mathematical properties of this distribution matches the underlying scientific assumptions, as the
decline is not as rapid as that for the Gompertz model and doesn’t appear to ha
under ve the large right
(CTH) Care.
hand tail of the exponential model. By 10 years of follow-up, cumulative survival is about 55%, so a
large proportion of the patients in the group have succumbed to AATD. An illustration of the fit of
the models compared with digitised survival data is presented in Figure 17. Aged
released 1982
s45, s47(1)(b)
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
A summary of the goodness-of-fit statistics (Akaike’s Information Criteria—AIC) reported for each of
the distributions is presented in Table 56.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
93
Page 107 of 218
FOI 5155 - Document 4
Table 56
Goodness of fit and parameters for FEV 1 <50 no decline survival models
Parametric model
AIC
Shape
Scale
Other
(meanlog) (mu)
(sdlog) (sigma)
(Q)
Weibull
17.82
3.6440
11.6210
Exponential
18.31
0.046
Lognormal
17.86
2.389
0.457
Generalised gamma
19.86
2.39E+00
4.54E-01
2.43E-02
Gompertz
17.83
4.79E-01
2.45E-03
Log-logistic
17.94
3.89
10.85
Abbreviations:
AIC = Akaike’s information criteria.
FEV1 <50 slow decline
the
An illustration of the fit of the models compared with digitised survival data is presented in Figure
18, using analysis provided by s47G
(2017).
s45, s47(1)(b)
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
s45, s47(1)(b)
As for other
survival extrapolations, sensitivity analysis provided at the end of Section D includes use of all of the
models to gauge sensitivity of the estimated ICER to the parametric model.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
94
Page 108 of 218
FOI 5155 - Document 4
s45, s47(1)(b)
FEV<50 rapid decline
the
Dawkins et al. (2009) provided Kaplan-Meier plots of cumulative hazard for mortality across nine
years of follow-up for the UK AATD registry. When categorised by FEV1% predicted, “the group with
severe impairment had increased mortality (pZ<0.001) compared with the mild group and there was
under
a direct relationship between severity and mortality. Cox regression analyses indicat
(CTH) ed that
Care. these
relationships remained when corrected for age.” (ibid, p. 1540). s45, s47(1)(b)
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
95
Page 109 of 218
FOI 5155 - Document 4
s45
the
under
(CTH) Care.
C.4. 4
RELATIONSHIP OF PRE-MODELLING STUDY TO THE ECONOMIC EVALUATION
Aged
released 1982
Patient data from the four years of RAPID/RAPID-OLE are used to model AT transition during the trial
Act and
period, and two years for BSC. A stepped analysis is included, where benefits are extrapolated from
Year four for 26 years (30-year total model projection) to reflect a patient lifetime. Patients are
been
assumed to stay on no decline, slow and rapid decline tracks for the remaining 26 years of the
projection. Annual mortality during the
has RAPID trial is used fo
Health r the trial period, after which
extrapolation of survival is undertaken using parametric
of models fitted to the UK registry data using
Information
the analysis by CSL Behring (2017). For consistency, the Gompertz model was used for all health
of
states, as this model had the best fit across most sub-populations. Different models are included in
sensitivity analyses. document
C.5
RELATIONSHIP OF EACH PRE-MODELLING STUDY TO THE ECONOMIC EVALUATION
This Freedom
Department
Section C included three pre-modelling studies addressing issues related to applicability and
the
extrapolation, which have implications for the economic model presented in Section D. A summary
by
of the results and implications is provided in Table 59.
Table 59
Summary of results of pre-modelling studies and their uses in the economic evaluation
Section
Pre-model ing
Results used in
Cross-
Results used in
Cross-
study
Section D
reference sensitivity analyses reference
Applicability of the
The population described in
trial-based evidence
Section B is the same as
Section
to the proposed
Study C.2
that used in the economic
D.2.
NA
NA
MBS population
model.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
96
Page 110 of 218
FOI 5155 - Document 4
FEV1 stratified utilities were
Selection of utilities
Study C.3
taken from the UK registry
Section
and applied health states
D.4. 1.
with FEV1<50 and FEV1>50
Extrapolation of trial-
Extrapolation from 4-year
Section
based evidence
Study C.4
trial period for 30 years in a
stepped analysis.
D.4.1.
NA
NA
Abbreviations:
FEV1 = forced expiratory volume in 1 second,
MBS = Medicare Benefits Schedule,
NA = not applicable.
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
97
Page 111 of 218
FOI 5155 - Document 4
SECTION D
ECONOMIC EVALUATION
D.1.
OVERVIEW
A1PI maintenance therapy has the aim of slowing the progression of emphysema in adults with
AATD (A1PI<11μM). An economic evaluation has been undertaken in this assessment using a cost-
utility approach. The model estimates cost per year of life and cost per QALY as an incremental cost-
effectiveness ratio (ICER). The model compares A1PI AT with optimal pharmacologic and non-
pharmacologic treatment (BSC), compared to BSC alone. Lung transplantation is not included as a
comparator, however, it is included in the clinical pathway for each arm of the model. A proportion
the
of patients who reach FEV1<50 with rapid or slow decline CT-measured lung density are assumed to
receive lung transplantation.
under
Results of the economic model are presented in two steps. The first step outlines cost-effectiv
Care. eness
(CTH)
results for the trial period of four years. This length of follow-up reflects maximum follow-up of the
RAPID trial (Chapman et al. 2015) and the open-label extension study (RAPID-OLE) (McElvaney et al.
1982 Aged
2017) trials. An average hypothetical cohort of 1,000 patients progresses between FEV1% and CT-
released
measured lung density decline states based on results of the trial within a cohort-based semi-
Act and
Markov model. Mortality data were taken from the RAPID-OLE and RAPID studies for the first two
and four years, respectively (McElvaney et al. 2017); (
been Chapman et al. 2015).
Health
The efficacy benefit associated with treatm
has ent that leads to improvements in patient morbidity are
captured in the model using RAPID trial data, with th
of e primary analysis being expressed as the
Information
incremental cost per additional QALY gained. Resource use is attached to each state using proposed
of
A1PI maintenance therapy product costs and MBS item costs. Australian Refined Diagnosis Related
Groups (AR-DRG) costs are applied to the frequency of GP and hospital presentations for UK COPD
document
patients of differing severity (Thomas et al. 2014) to estimate AATD disease management costs.
Department
The second step inv
This olves extrapolating RAPID transition data over an additional 26 years (lifetime). It
Freedom
was assumed that transitions between health states with varying rates of lung density decline
the
occurred during the follow-up of the RAPID and RAPID-OLE studies and that patients stayed on no,
by
slow or rapid decline tracks for the remaining 26 years. Mortality data for the remainder of the
model’s lifelong time-horizon were based on observations from 10 years of followed-up patients in
the UK AATD registry. A number of parametric models were fitted to the UK registry data to
extrapolate observational data for the lifetime projection. A range of sensitivity analyses were
undertaken to test the robustness of the results of the modelled economic evaluation. This includes
changes in baseline distributions of individuals with emphysema or COPD stratified according to
airflow obstruction, being mild, moderate, or severe.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
98
Page 112 of 218
FOI 5155 - Document 4
D.2.
POPULATIONS AND SETTINGS
D.2.1.
POPULATION
The modelled patient population is aligned with the proposed listing. Patient-level data was derived
from the RAPID trial with eligible patients having AATD (A1PI<11μM), FEV1 of 35%–70% (predicted)
and being non-smokers. The PICO notes patient eligibility as being ex- or never-smoking individuals
with severe A1PI deficiency (serum levels ≤11 μM) and emphysema with FEV1 <80%. The inclusion
criteria for the RAPID trial largely corresponds with the listing PICO, although a FEV1 of 35%–70%
(predicted) was included in the RAPID trial compared to FEV1 <80% for the proposed listing (See
Table 60).
the
Table 60
Comparison between eligibility criteria in the RAPID study and circumstances of use
Characteristic
RAPID Study
Proposed listing
under
(CTH) Care.
Inclusion criteria
Severe A1PI deficiency (serum concentration A1PI deficiency (serum levels ≤11 μM) and
<11μM) with a FEV1 of 35–70% (predicted).
emphysema with FEV1 <80%.
Aged over 65 years;
1982 Aged
Smoked tobacco within 6 months before
released
Exclusion criteria
recruitment;
Smokers (only ex- or never-smoking individuals
Act and
Waiting list or previous lung transplantation,
eligible)
lobectomy, or lung volume-reduction surgery; or
been
selective IgA deficiency.
Dose regimen
Intravenously 60mg/kg
Intravenously 60mg/kg
has
Health
Treatment
of
frequency/duration
Once per week up to 48 months
Once per week
Information
Abbreviations =
A1PI = alpha-1 proteinase inhibitor,
FEV1 = forced expiratory volume in 1 second,
μM = micromolar.
of
Baseline patient data from the RAPID trial informs cohort age and disease severity at entry into the
model, whilst a post-hoc analysis of Individual Participant Data (IPD) was performed by CSL Behring
document
(2017) to produce transition matrices informing health state transitions over the trial period. Trial
data are available to four years, after which the av
Department ailable within-trial data are extrapolated over a
This Freedom
lifetime (further 26 years).
the
The distribution of patients across health states at the beginning of the model (Table 61) was based
by
on the first year BSC patient profile from the RAPID study (Table 6; Table 7). s45, s47(1)(b)
. Based on the RAPID data,
the baseline age was assumed to be 53 years. This variable is relevant to length of the lifetime
projection and has no impact on the treatment effectiveness or natural history of the disease in the
model.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
99
Page 113 of 218
FOI 5155 - Document 4
Table 61
Baseline disease severity – RAPID population; baseline disease severity in the model
Baseline FEV1 and lung density
AT
BSC
BSC
decline
N= 85 (death 2)
(%)
RAPID
FEV1>50 no decline
N (%)
Not reported
6
7%
FEV1>50 slow decline
N (%)
Not reported
11
13%
FEV1>50 rapid decline
N (%)
Not reported
17
20%
FEV1<50 no decline
N (%)
Not reported
7
8%
FEV1<50 slow decline
N (%)
Not reported
29
34%
FEV1<50 rapid decline
N (%)
Not reported
15
18%
Model ed population
FEV1>50 no decline (%)
(%)
BSC from RAPID
7%
the
FEV1>50 slow decline (%)
(%)
BSC from RAPID
13%
FEV1>50 rapid decline (%)
(%)
BSC from RAPID
20%
FEV1<50 no decline (%)
(%)
BSC from RAPID
8%
under
FEV
Care.
1<50 slow decline (%)
(%)
BSC from RAPID
34%
(CTH)
FEV1<50 rapid decline (%)
(%)
BSC from RAPID
18%
Abbreviations:
BSC = best supportive care,
FEV1= forced expiratory volume in 1 second.
1982 Aged
Survival models were derived from UK registry populations. The RAPID and UK registry populations
released
appear to have similar characteristics. The average patient age in the UK was 52 years, with males
Act and
comprising 58% of the population (Green et al. 2016). FEV1 (% predicted) was 45.3%, which is slightly
lower than that reported for baseline patients in
been the RAPID trial of 47.4% and 47.2% for the
intervention and comparator arms, respectively. Baseline density (g/l) in the UK registry was 46.2,
has
Health
compared to 45·5 and 48·9 on each of the RAPID arms.
Information
of
D.2.2.
SETTINGS
of
The economic model assumes an Australian health care setting, with the modelled population
representing adults with documented severe A1PI deficiency. The RAPID study from which much of
document
the data is derived is relevant and applicable to this setting. The RAPID trial was global and included
a small number of Australian patients. Australia
Department contributed 9.7%-12.6% of patients to the
This Freedom
intervention and comparator arms, respectively, while Europe contributed 32.3%-27.6%, North
the
America 25.8%-25.3%, and Nordic countries 32.3%-34.5%. Dosing fol owed that recommended for
by
maintenance therapy of 60mg/kg by once-weekly infusion (Zemaira product information,
PROLASTIN-C product information; Appendix 1.). This infusion would be provided in an outpatient
setting.
The administration cost of $65.05 for each infusion was based on MBS item number 13915. It is
noted that patients could possibly be trained to self-infuse at home, which would reduce
administration costs. This possibility is included as a sensitivity analysis (see Section D). It does not
have a significant impact on the estimated ICER, but would potential y help with convenience and
overall patient adherence. Assumed average weight in the RAPID study of 76kg was used to estimate
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
100
Page 114 of 218
FOI 5155 - Document 4
product costs, resulting in an average use of 4.55 vials (1,000 ml) per week. This was multiplied by
the average compliance from the RAPID study of 93.9%, resulting in an average use of 4.28 vials per
week (Chapman et al. 2015). Fractional vials are rounded to whole numbers (i.e. five vials) and vial
usage per week is multiplied by 52 to estimate annual product usage costs. AT product costs account
for more than 90% of the estimated resource use in the lifetime model and is the key driver of the
calculated ICER.
BSC encompasses a range of interventions including pharmacological (e.g. bronchodilators, systemic
corticosteroids), non-pharmacological (e.g. oxygen therapy), and preventative measures such as
vaccinations. AATD disease management costs are derived from Thomas et al. (2014). This study was
a retrospective, observational study undertaken in 10 General Practices in England, using routine
the
clinical records of 511 patients with COPD. It reported the frequency of hospital and GP visits
stratified by COPD severity, based on each patient’s FEV1 measurement recorded during 2007. The
study included 314 (61%) mild-moderate patients (≥50% predicted FEV1), 145 (28%) severe (30–49%
under
predicted FEV
Care.
1) and 52 (10%) very severe (<30% predicted FEV1) patients. Costs by severity are
(CTH)
applied to patients who transition through the Markov model according to probabilities estimated
for the intervention and comparator arms. Differences in costs are captured in the incremental cost
1982 Aged
per QALY calculation, which summarised cost-effectiveness results.
released
Act and
D.3.
STRUCTURE AND RATIONALE OF THE ECONOMIC EVALUATION
been
A cost-utility analysis was undertaken to determine the value of AT in addition to optimal
pharmacological treatment and supportive care. Table 62 summari
Health ses the key characteristics of the
has
economic evaluation.
Information
of
Table 62
Summary of the economic evaluation
of
Perspective
This economic evaluation was conducted from the perspective of the Australian health
system. It includes resource use supported by government and patients, along with
document
health outcomes applicable to the treatment of patients with emphysema due to A1PI
deficiency.
Department
Intervention
Augmentation therapy in addition to optimal pharmacological treatment and supportive
This Freedom
care.
the
Comparator
Best Supportive Care. Optimal pharmacological treatment and supportive care
Type of economic evaluation
Cost-utility analysis
by
Sources of evidence
RAPID study, RAPID-OLE study, UK Registry data
Time horizon
30-year time horizon in the base case
Sensitivity analyses include a time horizon of 20 years and 40 years
Outcomes
Quality-adjusted life years/ life-years gained
Methods used to generate
Cohort expected value analysis
results
Health states
1.
FEV1≥50% predicted, no lung density decline
2.
FEV1≥50% predicted, slow lung density decline
3.
FEV1≥50% predicted, rapid lung density decline
4.
FEV1<50% predicted, no lung density decline
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
101
Page 115 of 218
FOI 5155 - Document 4
5.
FEV1<50% predicted, slow lung density decline
6.
FEV1<50% predicted, rapid lung density decline
7.
Lung transplant
8.
Dead
Cycle length
1 year
Discount rate
5% used for base and 3.5% and 7% sensitivity analyses
Software packages used
Microsoft Excel 2010
Abbreviations: A1PI = alpha-1 proteinase inhibitor;
FEV1 = forced expiratory volume in 1 second.
As noted, a stepped evaluation was undertaken. The first step captures costs and health outcomes
over four years, the maximum number of years of follow up in the RAPID-OLE trial. AT is expected to
have longer term benefits such as prolonging life and delaying the need for lung transplantation.
Correspondingly, a lifetime extrapolation is also included as a second step. The extrapolation is
the
carried forward over 26 years, thus the overall modelling period is 30 years.
This timeframe is selected to align with the patient population in the RAPID trial. The age at baseline
under
was 53.8 years in the RAPID trial (Chapman et al. 2015). Average life expectancy in Australia
Care. for a
(CTH)
male aged 54 is 28.9 years, and 32.1 years for females (ABS 2016). Sensitivity analyses were
conducted for scenarios where the time horizon was varied to 20 years and 40 years. The ICER is
1982 Aged
relatively insensitive to these changes as a large proportion of the patients in each arm of the model
released
are expected to suffer mortality within the first 20 years of the projection.
Act and
D.3.1.
LITERATURE REVIEW
been
A1PI deficiency
has
Health
of
A literature review was conducted in June 2018 using the search terms provided in Table 63 to
Information
identify cost-effectiveness studies for treatment of A1PI deficiency. The search included EMBASE
of
(1947-), other HTA websites (Canadian Agency for Drugs and Technologies in Health—CADTH; Health
Technology Assessment—HTA, National Institute for Clinical Excellence—NICE) and the Cochrane
document
Library.
Department
Table 63
Search
This
terms used
Freedom
Element of clinical question
Sear
the
ch terms
AATD.mp OR
by alpha-1 antitrypsin deficiency.mp OR
Population
antitrypsin deficiency.mp OR
proteinase inhibitor OR Prolastin OR Aralast OR Zemaira OR Trypsone
Intervention
Not applicable
Comparator (if applicable)
Not applicable
Outcomes (if applicable)
Not applicable
Health economics OR economic aspect OR economics OR biomedical technology
Other
assessment OR economic evaluation OR health care cost OR technology assessment
OR cost ef ectiveness analysis OR cost minimisation analysis OR cost minimization
analysis OR cost minimization analysis OR cost utility analysis
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
102
Page 116 of 218
FOI 5155 - Document 4
Element of clinical question
Search terms
English language
Limits
Remove duplicates
1990-2018
Table 64
Summary of the process used to identify and select studies for the economic evaluation
EMBASE
Other HTA
Cochrane
websitesa
Library
Number of titles and abstracts reviewed after search
1073
TOTAL number of exclusions
1062
Number of HTA reports/cost-effectiveness outcomes reported
11
2
Consolidated number of studies excluding duplicates
13 the
a HTA agencies included: NICE, CADTH.
Abbreviations: CADTH, Canadian Agency for Drugs and Technologies in Health; HTA, Health
Technology Assessment, NICE, National Institute for Clinical Excellence
under
(CTH) Care.
Of 113 studies screened, only five published economic studies for A1PI deficiency treatment were
identified. Table 65 lists the publications included in the review of economic evaluations.
1982 Aged
Table 65
Economic models assessing A1PI deficiency treatment
released
Published economic models assessing A1PI defi
Act
ciency treatment
and
Study
Reference
been
Hay and Robin
Hay JW, Robin ED. Cost-effectiveness of alpha-1 antitrypsin replacement therapy in treatment of
1991
congenital chronic obstructive pulmonary disease. Am J Public Health 1991; 81:427–433
has
Health
Alkins and
Alkins SA, O’Malley P. Should health-care systems pay for replacement therapy in patients with alpha
O’Malley 2000
(1)-antitrypsin deficiency? A critical review and c
of ost-effectiveness analysis. Chest 2000; 117:875–880
Information
Gildea et al. 2003 Gildea, et al. 2003. Cost-effectiveness Analysis of Augmentation Therapy for Severe Alpha 1-
Antitrypsin Deficiency. American Journal of Respiratory and Critical Care Medicine, pp. 1387-92
of
Shermock et al.
Shermock KM, Gildea TR, Singer M, Stoller JK. Cost-effectiveness of population screening for alpha-1
2005
antitrypsin deficiency: a decision analysis. COPD. 2005;2: 411–8
document
Sclar DA, Evans MA, Robison LM, Skaer TL. Alpha1-Proteinase inhibitor (human) in the treatment of
Sclar et al. 2012
hereditary emphysema secondary to alpha1-antitrypsin deficiency: number and costs of years of life
Department
gained. Clin Drug Investig. 2012; 32:353–60
This Freedom
Other economic studies
the
Mullins et al.
Mullins CD, Huang Z, Merchant S, et al. The direct medical cost of alpha (1)-antitrypsin deficiency.
2001
Chest 2001;
by 119: 745-52
Mullins et al.
Mullins CD, Wang J, Stoller JK. Major components of the direct medical costs of alpha1-antitrypsin
2003
deficiency. Chest. 2003; 124:826–31
Barros-Tizón JC, Torres ML, Blanco I, Martínez MT; Investigators of the rEXA study group. Reduction
Barros-Tizón et
of severe exacerbations and hospitalization-derived costs in alpha-1-antitrypsin-deficient patients
al. 2012.
treated with alpha-1-antitrypsin augmentation therapy. Ther Adv Respir Dis. 2012 Apr;6(2):67-78. doi:
10.1177/1753465812438387. Epub 2012 Feb 21
Campos. et al.
Campos, M. et al. 2015. Utilization and Costs Associated with the Prolastin Direct Alpha 1 Proteinase
2015
Inhibitor Patient Management Program. Obstructive Lung Diseases, October 2015
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
103
Page 117 of 218
FOI 5155 - Document 4
Published economic models assessing A1PI deficiency treatment
Study
Reference
Zacherle et al.
Zacherle, JM Noone, MC Runken, CM Blanchette 2015. PSY35 - Health Care Cost and Utilization
2015
Associated with Alpha-1 Antitrypsin Deficiency Among a Cohort of Medicare Beneficiaries with COPD.
Value in Health, Volume 18, Issue 7, November 2015, Page A664 E
Karl et al. 2017
Karl, F. et al. 2017. Costs and health-related quality of life in Alpha-1-Antitrypsin Deficient COPD
patients. Respiratory Research 2017 18:60 DOI 10.1186/s12931-017-0543-8
CADTH 2017
Alpha1-Proteinase Inhibitors for the Treatment of Alpha1Antitrypsin Deficiency: A Review of Clinical
Effectiveness, Cost Ef ectiveness, and Guidelines
NICE 2010
Human alpha1-proteinase inhibitor for treating emphysema ID856, in development [GID-HST10017]
Expected publication date: 13 February 2019
Abbreviations:
COPD = chronic obstructive pulmonary disease.
the
Hay and Robin 1991
Hay and Robin (1991) undertook a cost-effectiveness analysis of AT costs relative to years of life
under
saved. Clinical benefits were based on 14 years of disease progression follow-up in 246 Swedish
(CTH) Care.
AATD patients, applied to the adult US population. A non-smoking 50-year-old male was estimated
to have an additional 7.12 years life expectancy with AT. The cost per life year estimates ranged
1982 Aged
from US$28-39 thousand for smoking males and females, and US$41-72 thousand for non-smokers.
released
The relatively cost-effective results appear to be driven by the estimated increase in life expectancy
Act and
associated with AT. The base model assumed life expectancy increases of around 14 years and seven
years for non-smoking 50-year-old women and men, respectively. The non-smoking male life
been
expectancy benefit is double that estimated in this assessment of AT benefits in Australia of three
Health
years (in this assessment) for someone in th
has eir early 50s using RAPID and UK AATD registry data.
of
Alkins and O’Malley 2000
Information
of
Cost effectiveness analysis was undertaken for AT replacement therapy among individuals with
severe COPD (FEV1 < 50% of predicted). The study examined a payer perspective based on Medicare
document
reimbursement rates. Annual AT costs were estimated to be US$51,948 based on a dosage of
Department
60mg/kg, meaning a 70-kg patient would require nine 500-mg vials each week. A systematic review
This Freedom
of AT effectiveness studies in MEDLINE and EMBASE between 1980-1998 was used to define
the
absolute risk differences. NIH Registry data was identified that found the five-year mortality rate in
by
patients (FEV1 < 50%) ranged from 33% in the BSC arm to 15% in the AT arm. The incremental cost
per year of life saved for AT replacement therapy was US$13,971. The large gain in life expectancy
underpins this result. The authors noted that results depend substantially on the mortality rate
reduction. When the effect size was changed from 10% to 70%, the incremental cost per year of life
saved ranged from US$152,941 to US$7,330.
Gildea et al. 2003
Gildea et al. 2003 developed a five-state Markov model, based on FEV1% predicted, to compare AT
with BSC. The analysis took a health care system perspective. Health states were defined as FEV1
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
104
Page 118 of 218
FOI 5155 - Document 4
50% to 79% predicted, FEV1 35% to 49% predicted, FEV1 below 35% predicted, lung transplantation,
and death. Patients transitioned through the model based on probabilities largely derived from the
NHLBI Registry (AATD Registry Study Group, 1998).
It was assumed that 19% of patients with an FEV1 below 15% predicted underwent lung
transplantation. The model population had a baseline age of 46 years, 50% male and a FEV1 of 49%
predicted. Except for age, these characteristics are similar to that in the RAPID trial. Resource usage
was assigned to each state using a retrospective cost analysis of COPD management in the USA
(Hilleman et al. 2000). Resources included the cost of medications, oxygen therapy, laboratory and
diagnostic tests, clinic and emergency department visits, and hospitalizations. The annual cost of
Stage I COPD was US$1,966, Stage II COPD US$5,892 and Stage III COPD US$12,647. Transplantation
the
and post-transplantation resources were taken from cost studies of lung transplant patients
(Sharples et al. 2001, Ramsey et al. 1995), where the cost of lung transplantation was estimated at
US$328,222, along with annual maintenance costs of US$59,918. The annual cost of AT was
under
calculated to be US$54,765.
(CTH) Care.
Pulmonologists who treat AAT deficiency (n=14) were surveyed using the health utilities index (Mark
III) (Crystal et al. 1989) to estimate utility values. The utility assigned for
1982 Stage I C
Aged OPD was 0.93,
Stage II COPD 0.75 and Stage II COPD 0.26. The economic model c
released ompared AT with no AT, AT for
Act and
life, and using AT when FEV1 falls below 35% predicted. The ICER was $207,841/QALY for AT until
FEV1 is below 35% predicted and $312,511/QALY for the AT strategy.
been
Shermock et al. 2005
has
Health
of
The Shermock study used the same model as that in Gildea et al. 2003, however, it was extended to
Information
assess the economic attractiveness of AATD screening strategies in all newborns, in all 10-year-old
of
children, and no screening for PI∗ZZ AAT deficiency. The benefit of screening was that information
about presence of AATD resulted in a lower likelihood of smoking. Screening all newborns had an
document
ICER of US$422,000 per QALY gained.
This
Department
Sclar et al. 2012 Freedom
the
Sclar and colleagues used Monte Carlo simulation to estimate the number of years of life gained,
by
and health service expenditures per year of life gained, for AT. The authors formulated algorithms
(regression model) for the annual decline in FEV1, predicted FEV1 and mortality, from data held by
the AATD Registry Study Group (AATD Registry Study Group 1998, McElvaney et al. 1997).
Patients were deemed eligible for AT when predicted FEV1 fell below 70%. Expenditures for AT were
estimated using a dose of 60mg/kg, a once-weekly administration schedule, and an administration
fee of US$36.73 (Mullins et al. 2001). Patients were eligible for lung transplant when predicted FEV1
was below 40% (Crapo et al. 1981). The annual probability of lung transplant when eligible was
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
105
Page 119 of 218
FOI 5155 - Document 4
assumed to be 0.155 (Larsson 1978, Stol er et al. 1994). The cost of lung transplantation was
US$67,000 per year for three years.
AT therapy was estimated to result in a significant increase in years of life. Female non-smokers
gained an average of 9.19 years, at a cost of US$160,502 per year, and male non-smokers gained
10.60 years at US$59,234 per year. Again, these increases in life expectancy for AT therapy are
higher than those estimated in this assessment using RAPID trial data and parametric models fitted
to UK registry data.
Mullins et al. 2001
Mullins and colleagues undertook a literature search on the pharmacoeconomics of AAT deficiency
the
in Medline, Health STAR, International Pharmaceutical Abstracts and Ovid. Keywords included ‘α1-
antitrypsin deficiency’, ‘late diagnosis’, ‘early diagnosis’, ‘aerosol therapy’, ‘product shortage’,
‘epidemiology’ and ‘economics’. A total of 106 papers were identified and col ated on the basis of
under Care.
being ‘of interest’, ‘considerable interest’, ‘good overview of clinical aspects of AA
(CTH) T-deficiency’,
‘addresses the impact of FEV1 values’, ‘cost-effectiveness analysis of AAT deficiency replacement
therapy’, ‘burden of illness of AAT deficiency’ and ‘describes potential for aerosol augmentation
1982 Aged
therapy’. The papers by Hay and Robins (1991) plus Alkins and O’Mal ey (2000) were identified in the
released
economics section. The issue of the lack of statistical power gi
Act ven the rare nature of AATD was
and
noted. The review revealed a limited economic evidence base, with key papers provided in this
been
assessment.
has
Health
Mullins et al. 2003
Information
of
Mullins et al. performed a cost analysis on AATD patients in 1998 using a mail survey. Responses
of
were collated from 292 of the 688 individuals. The response rates for PI*ZZ individuals was 42.7%
versus 41.8% for non-PI*ZZ subject. The mean age of subjects was 52.0 years. The average costs per
document
year for hospital and outpatient services were US$4,497 and US$2,299. Prolastin and other
medicines cost $28, 075 and S6,456 per year. The Australian and US health settings are different, as
This
Department
one is insurance-based and the oth
Freedom er largely involves publicly delivered services. These results are of
limited applicability to the curre
the nt assessment
by
Barros-Tizón et al. 2012
This observational study was undertaken in Spain to evaluate the effect of 18 months of AT therapy
in reducing the incidence of exacerbations. The numbers of mild and severe exacerbations were
compared and hospitalization-related costs estimated. The total of 127 patients had an average age
of 51.7 years and 63.3% were male. “The average number of days of admission was 3.9 before
treatment and 3.0 in the treatment period, while in the population with exacerbations the values
were 6.7 and 4.6 days, respectively” (ibid, p. 74). Hospital costs were reduced by €416.76 per subject
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
106
Page 120 of 218
FOI 5155 - Document 4
in the total patient population after 18 months of AT augmentation therapy. The AT arm in RAPID
was not associated with a reduction in exacerbations.
Campos et al. 2015
The Campos analysis assessed the costs of 213 patients in the Prolastin Direct A1PI Patient
Management Program compared with 232 patient costs of augmentation therapy with other alpha-
1-proteinase inhibitors A1PI. The Prolastin Direct program was noted by the authors as a program
providing coordinated augmentation therapy services (reimbursement, pharmacy, infusion) in
conjunction with Prolastin. Similar demographic patient characteristics too RAPID were observed.
Patients were 51% male with mean age of 55.5 years. Mean total monthly costs were US$ 11,705 for
PD patients and US$ 13,803 in the comparator. Mean monthly augmentation therapy costs were
the
$9,901, therefore AT comprised 70%+ of total health care costs.
Zacherle et al. 2015
under
(CTH) Care.
Zacherle and colleagues assessed one-year patient costs in the USA following confirmed COPD or
AATD diagnosis for AATD-COPD patients (n=279) and COPD patients (n=183,832) using 2011-2013
1982 Aged
Medicare data. Mean age of COPD and AATD cohorts were 72.6 years and 64.6 years, respectively.
released
AATD patients presented more frequency in ER and as inpatient, with total healthcare costs (per
Act and
patient) being US$ 27,674 higher than COPD total costs. It was noted that AATD patient costs were
not adjusted for differences in COPD severity. Many o
been f the AT economic models use COPD severity
to cost resource use. Sensitivity analysis, which includes changed resource-cost assumptions for
has
Health
FEV1-related health states used in the base analysis, is included in the concluding section of this
of
economic assessment. Changes do not have a large impact on the estimated ICER, as the majority of
Information
resource use is associated with AT product costs.
of
Karl et al. 2017 document
Karl and colleagues calculated direct and indirect patient costs, and QoL using the German
Department
multicentre COPD
This cohort COSYCONET study (German COPD and Systemic Consequences –
Freedom
Comorbidities Network). Health-related QoL (HRQL, as assessed by SGRQ, CAT, and EQ-5D-3 L) was
the
compared between 131 AATD and 2,049 COPD patients. AATD patients were younger (60.3 years vs
by
65.4 years), more of them never smoked (23.7% vs 5.4%) and they were in higher GOLD grades than
the COPD patients. The association of AATD with costs and QoL was examined using generalised
linear regression modelling (GLM) adjusted for age, sex, GOLD grade, BMI, smoking status, education
and comorbidities. The costs of AT products were excluded.
The regression analysis found that AATD patients (with and without AT), tended to have lower total
costs compared to COPD patients without AATD, but these differences were not statistical y
significant. These results differ to Zacherle et al. 2015 who found AATD-COPD patients had higher
costs than COPD patients. The higher number of outpatient visits for AT receiving AATD patients was
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
107
Page 121 of 218
FOI 5155 - Document 4
thought to be a result of infusions. These costs were not separated in Zacherle et al. 2015. Average
direct annual costs were €6,099 in AATD patients without AT, €7,117 in AATD patients with AT
(excluding AT medicines), and €7,460 in COPD patients without.
Participants completed the SGRQ, (Jones et al. 1992) the COPD Assessment Test (CAT) (Jones et al.
2011) and the generic EuroQol 5 dimensions (EQ-5D-3L) questionnaire including VAS. There were no
significant differences between groups regarding QoL. This result is in line with the study by Manca
et al. (2014) who found no QoL differences between AATD/COPD patients and COPD patients
without AATD.
CADTH, 2017
the
CADTH undertook a systematic review of RCTs comparing A1PI with placebo. They concluded that
the impact of A1PI on the rate of decline in FEV1 and rates of exacerbations is variable. They noted
that AT therapy has not been demonstrated to lead to an improvement in patient QoL compared to
under Care.
placebo. No studies met the inclusion criteria to address the cost effectiveness of
(CTH) A1PI for the
treatment of adults with AATD.
1982 Aged
NICE, 2018
released
Act and
NICE are currently undertaking a review of AT therapy effectiveness and guidelines. They note that
670 people in England have emphysema caused by AATD (Miravitlles et al. 2010) and about 540 of
been
these people (80%) will have clinically significant emphysema requiring treatment (NIHR Horizon
Health
Scanning Centre 2014). They note that
has the epidemiology, disease characteristics and disease
progression of emphyema in patients with AATD differs
of from that in usual COPD. Consequently, it is
Information
inappropriate to include AATD within the umbrella of what is generally termed 'usual' COPD.8
of
Chronic Pulmonary Obstructive Disease (COPD)
document
Only a limited number of economic models have been developed to assess AT therapy. Some use
COPD states for utility and disease-management c
Department ost calculations. The economic literature review
This Freedom
was expanded to include some recent COPD economic model ing reviews.
the
Boland et al. 2013 by
The review by Boland and colleagues (2013) aimed to identify cost-effectiveness studies of COPD
management programs. MEDLINE, the economic evaluation database of the UK National Health
System (NHS-EED) and the EUROpean Network of Health Economic Evaluation Database
8 https://www.nice.org.uk/guidance/gid-hst10017/documents/scope-consultation-comments-and-responses
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
108
Page 122 of 218
FOI 5155 - Document 4
(EURONHEED) were searched in July 2011. Sixteen papers describing 11 studies were identified.
Changes in health related QoL were reported using SGRQ in six studies. Five reported an improved
QoL, however, the reduction did not exceed the clinically relevant improvement of four points
(Jones et al. 2005). Three studies measured health-related QoL on a VAS and only one study
measured the EQ-5D.
Zafari et al. 2017
This study is the most recent published review. It included a systematic search for decision-analytic
modelling in COPD using MEDLINE, Embase, and citations within reviewed articles. The search
resulted in 4054 references, excluding duplicates. After full-text review, 49 publications met the
inclusion criteria. Decision trees and Markov models were the most popular approaches (43 studies)
the
and disease progression was model ed through clinical staging in most studies. A range of methods
was used to model COPD progression, some directly via the continuum of lung function (eg. FEV1),
others in discrete clinical states defined by the GOLD grades. Four Markov model
under s used exacerbation
(CTH) Care.
status in defining model states. In general, effectiveness was modelled as a direct reduction in
exacerbation rate.
1982 Aged
Forty of the models were developed for the purpose of economic evaluation, either of alterative
released
COPD treatments or of a COPD management program. The Marko
Act v model developed by Oostenbrink
and
et al. (2005) was the most widely adopted model structure, which has been used in eight subsequent
been
studies. Other widely adopted model structures were from Borg et al. (2004), Price et al. (2011),
Spencer et al. (2005), Hoogendoorn et al. (2005), Sin et al. (2004), Buist et al. (2005), and Asukai et
has
Health
al. (2013). The features of these widely adopted models were summarised as part of the European
of
consortium review outlined in Hoogendoorn et al. (2016).
Information
of
Hoogendoorn et al. 2016
document
The consortium of COPD model ing groups reviewed nine recent COPD economic models and
reported patient characteristics, disease progression, mortality, QALYs, and costs for hypothetical
This
Department
subgroups of patients. The consor
Freedom tium reviewed how they differed and how model outcomes for
exacerbations and mortality we
the re validated. Features of the models are summarised in Table 66.
by
Table 66
Summary of COPD economic model progression and mortality characteristics
Study
Disease progression
Exacerbation frequency
Mortality
specified by
specified by
specified by
Asukai Markov
Sex, age, smoking
FEV1% pred
Sex, age, FEV1% pred
Asukai simulation
Sex, age, smoking, FEV1
FEV1% pred
Sex, age, FEV1% pred
Borg
Age, FEV1, rapid decline
FEV1% pred, frequent
exacerbations
Age, FEV1% pred
Sex, age,
Briggs
Sex, age, smoking
Sex, age, smoking, FEV1%
pred
smoking,
FEV1% pred
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
109
Page 123 of 218
FOI 5155 - Document 4
all other listed
Age, RV, D
Sex, age, RV, D
LCO,
LCO, BODE,
Dal Negro
% FEV decline,
Age, RV, DLCO, BODE, %
BODE, % FEV
comorbidities
FEV decline, comorbidities
decline,
comorbidities
Hansen
Smoking, FEV1% pred
FEV1% pred
Age, FEV1% pred.
Hoogendoorn
Sex, age, smoking
FEV1% pred
Sex, age, smoking FEV1%
pred
Samyshkin
Sex, age, FEV1% pred
FEV1% pred
Sex, age, FEV1% pred
Wacker
Smoking, FEV
Sex, age, smoking, FEV1%
1% pred
FEV1% pred, lung
transplant
pred, lung transplant
Abbreviations:
BODE = body mass index, airflow obstruction, dyspnoea and exercise index,
DLCO = dif using capacity for carbon
monoxide,
FEV1 = Forced expired volume in 1 second,
pred = predicted,
RV = residual volume.
the
In all models except the Markov model of Asukai, disease progression was specified by FEV1%
predicted. Most of the nine models use a series of discrete COPD health states. For example, the
Asukai Markov has mild, moderate, severe, and very severe COPD states, based on pre-
under Care.
bronchodilator FEV
(CTH)
1 measures reported in the Indacaterol clinical trials, and death. Cut-off points
adopted to define severity were the same as for GOLD. Transition probabilities used in the model
were based on patient movement in trials. Borg also defined disease severity by lung function (FEV1,
1982 Aged
as a percentage of predicted) divided into four different states using the GOLD guidelines. Hansen
released
developed an Excel-based Markov model with four health states
Act representing the Global Initiative
and
for Chronic Lung Disease (GOLD) disease severity classification. Within each GOLD stage, three sub-
been
states were included to model stable disease with three levels of COPD exacerbation.
has
Health
All models used FEV1% predicted to specify exacerbation frequency, mortality, utilities, and disease
of
management maintenance costs. Exacerbation costs were specified by FEV1% predicted by four
Information
models (Hoogendoorn et al. 2016). of
Lung transplant document
A literature review was conducted in June 2018 using the search terms ‘Lung transplant or lung
Department
transplantation’ an
This d economic terms provided in Table 63 to identify cost-effectiveness studies on
Freedom
treatments for lung transplantation. The search included EMBASE (1947-), other HTA websites
the
(Canadian Agency for Drugs and Technologies in Health—CADTH, Health Technology Assessment—
by
HTA, National Institute for Clinical Excellence—NICE) and the Cochrane Library. The studies
identified in the search are summarised in Table 67.
Table 67
Summary of the process used to identify and select lung transplant studies for the economic
evaluation
EMBASE
Other HTA
Cochrane
websites a
Library
Number of titles and abstracts reviewed after search
474
Total number of exclusions
468
Number of HTA reports/cost-effectiveness outcomes reported
6
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
110
Page 124 of 218
FOI 5155 - Document 4
EMBASE
Other HTA
Cochrane
websites a
Library
Consolidated number of studies excluding duplicates
6
Abbreviations: CADTH, Canadian Agency for Drugs and Technologies in Health; HTA, Health Technology Assessment, NICE, National
Institute for Clinical Excellence.
a HTA agencies included: NICE, CADTH.
Of 474 studies screened, only five published economic models for lung transplant were identified.
Table 65 and Table 68 list the publications included in the review of economic evaluations.
Table 68
Economic evaluations of lung transplantation
Published economic models assessing lung transplantation
Study
Reference
the
Al et al. 1998
Al MJ, Koopmanschap MA, van Enckevort PJ, Geertsma A, van der Bij W, de Boer WJ, et al. Cost-
effectiveness of lung transplantation in The Netherlands: a scenario analysis. Chest. 1998; 113:124-30
Ramsey et al.
Ramsey SD, Patrick DL, Albert RK, Larson EB,Wood DE, Raghu G. The cost-effectiveness of lung
under
1995
transplantation: a pilot study. University of Washington Medical Center Lung Transplant Study Group.
Chest. 1995;108: 1594-601
(CTH) Care.
Sharples et al.
Sharples LD, Taylor GJ, Karnon J, Caine N, Buxton M, McNeil K, Wallwork J. A model for analyzing
2000
the cost of the main clinical events after lung transplantation. J Heart Lung Transplant. 2001 Apr;20
(4):474-82.
1982 Aged
Vasiliadis et al.
Vasiliadis HM, Collet JP, Penrod JR, Ferraro P, Poirier C. A cost-effectiveness and cost-utility study of
released
2005
lung transplantation, J Heart Lung Transplant. 2005 Sep;24(9):1275-83.
Act and
Anyanwu et al.
Anyanwu AC, Rogers CA, Murday J. Where are we today with pulmonary transplantation? Current
2000
results from a national cohort. UK Cardiothoracic Transplant Audit Steering Group. Transpl Int.
been
2000;13 Suppl 1:S245-6
Groen, et al.
Groen, H., 2004. Cost-Ef ectiveness of Lung Transplantation in Relation to Type of End-Stage
Health
2004
Pulmonary Disease. American Jo
has urnal of Transplantation, 4(7), pp. 1155-62.
Other studies
of
Information
Paraskeva et al.
Paraskeva, M. et al. Lung transplantation in Australia, 1986-2018: more than 30 years in the making.
2018
MJA, 2018, Narrative Review (10) 4 June 2018
of
Al et al. 1998
document
Al et al. (1998) developed a microsimulation model based on Dutch lung transplantation data from
Department
1990 to 1995 to es
This timate QoL and costs with and without transplantation. The data set included 425
Freedom
patients referred for lung transplantation, 57 of whom underwent transplantation. The model
the
estimated survival with and without transplantation using a Weibull parametric model.
by
Health-related QoL was elicited using a self-administered questionnaire. It contained domains
covering wel being, depression, anxiety and daily activities, along with EuroQol and Nottingham
health profile generic instruments. Patients completed the questionnaire at outpatient screening
phase, then every three months. Following transplantation, QoL was measured at one, four, and
seven months, then every six months.
QALY were calculated from the EuroQol responses. Average patient utility on the waiting list varied
from 0.55 at six months or less, to 0.4 for more than 15 months. Utility of 0.52 at screening, declined
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
111
Page 125 of 218
FOI 5155 - Document 4
the longer that patients awaited transplantation. Utility increased after transplantation from 0.53 at
one to three months post-transplant, to 0.9 by 25 months or more. For the baseline scenario, the
costs per life-year gained are G194,000 (G = Netherlands guilders) and the costs per QALY gained are
G167,000.
Ramsey et al. 1995
Ramsey and colleagues undertook a cost-effectiveness analysis of lung transplantation among 25
patients in a pilot study in the USA. Inpatient and outpatient costs were identified from the hospital
billing service and home health agencies. QALY scores were computed using utility scores obtained
through SG interviews. The post-transplant mean utility score of 0.8 was significantly higher than the
mean waiting-list score of 0.68. Survival data was sourced from an international lung transplant
the
registry and from studies of patients on lung transplant waiting lists (Deng et al. 2009).
Transplantation cost was US$164,989, with fol ow-on post-transplant monthly costs of US$11,917.
Life expectancy was not significantly increased for lung transplant patients 5.8
under 9 years compared to
(CTH) Care.
waiting-list patients 5.32 years, although quality-adjusted life expectancy did improve. The
incremental cost per QALY gained for post-transplant compared with waiting-list patients was
US$176,817.
Aged
released 1982
Sharples et al. 2000
Act and
A Markov model was developed using a retrospective
been analysis of 359 patients in the UK to estimate
post-transplant complications patient costs. BOS was found to be a key cost driver. Acute events
has
Health
such as rejection and CMV infections also influenced cost. At the end of five years, 52.1% of the lung
of
transplant recipients had died.
Information
of
Vasiliadis et al. 2005
Lung transplantation cost-effectiven
document ess analysis was undertaken using 124 patients in Quebec
between 1997 and 2001. Survival was presented in mean life-years and utility was assessed by the
Department
standard gamble a
This pproach (Von Neumann 1944) across 34 candidates and 71 recipients. During the
Freedom
interview, the standard gamble was supplemented with the use of a probability wheel (Torrance
the
1976). The average utility assigned to the post-transplant period was calculated yearly up to year
by
four and an average score applied for beyond four years. For 37 patients, the annual mean
difference utilities were 0.63, 0.7, 0.48, 0.77 and 0.45. The waiting list average utility was 0.17. Forty
of the transplants were single and 36 double. The mean life years and QALYs gained were 0.57 and
0.62, respectively. The cost per patient without transplantation was US$1,102 per month. The cost
of the transplant was US$31,815, with a four years average post-transplant cost per month around
US$1,156 (range from US$626 to US$1,809).
Anyanwu et al. 2000
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
112
Page 126 of 218
FOI 5155 - Document 4
This UK economic analysis included data from seven transplantation units, and survival data from
677 lung transplants, which is higher than some of the earlier cited studies. Data from the national
UK Cardiothoracic Transplant Audit database (Anyanwu et al. 2000) was used to compute survivals.
Four-year national survival data were extrapolated to 15 years by using parametric modelling.
Survival gain in this study (from 2.0 to 2.5 years for 15 years) was less than that found in the Dutch
study (Van Enckovert et al. 1998), which yielded a gain of 4.4 years.
Health utility and health-related quality of life (HRQoL) were measured using the EQ-5D
questionnaire (Anyanwu et al. 2001). The survey was undertaken across 87 patients waiting for lung
transplantation and on 255 transplant recipients. These utilities were described earlier. The average
costs were $176,640, $180,528, and $178,387 for single-lung, double-lung, and heart-lung
the
transplantation. The ICERS were $48,241 for single-lung, $32,803 for double-lung, and $29,285 per
QALY gained for heart-lung transplantation.
Across a 15-year period, lung transplantation yielded mean benefits (relative to
under medical treatment)
(CTH) Care.
of 2.1, 3.3 and 3.6 QALY for single-lung, double-lung and heart-lung transplantation, respectively.
During the same period, the mean cost of medical treatment was estimated at $73,564, compared
with $176,640 for single-lung, $180,528 for double-lung, and $1
1982 78,387
Aged for heart-lung
transplantation. The costs per QALY gained were $48,241 for single
released -lung, $32,803 for double-lung,
Act and
and $29,285 for heart-lung transplantation. been
Groen et al. 2004
has
Health
A microsimulation model was developed using performance data from the lung transplantation
of
program in the Netherlands. There was variation in ICERs between interventions. The variations
Information
were driven by differences in survival and in quality of life. ICERS varied from 77 to 90 at a 5%
of
discount rate. The authors concluded “as a result of relatively small numbers of patients, the
confidence intervals of survival estimates for some diagnoses are rather wide. Despite this limitation
document
in the source data, closely fitting survival functions could be constructed for all diagnoses “(ibid, p.
1161).
This Freedom
Department
Paraskeva et al. 2018 the
by
Paraskeva and colleagues reviewed lung transplant waiting times and survival in Australia in early
2018. They noted that lung transplantation remains limited by donor supply, with 15%-20% of lung
transplant candidates dying while on a waitlist. Age-related eligibility was highlighted as a
consideration, with candidates of more than 65 years being considered with minimal comorbidities.
Most lung transplants in Australia are bilateral, with 203 conducted in 2016 (Australia and New
Zealand Cardiothoracic Organ Transplant Registry. 2016) Survival of bilateral lung transplant
recipients at one, three and five years has been estimated at 90%, 74% and 68%, respectively, which
are higher than international survival rates of 82%, 69% and 59% (Chambers et al. 2017). Given that
Australian survival is higher; a sensitivity analysis is included with lower rate of mortality following a
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
113
Page 127 of 218
FOI 5155 - Document 4
lung transplant. Given that only a small proportion of patients receive transplantation, this
sensitivity analysis has a limited impact on the estimated ICER.
Summary
Only a limited number of economics studies relating to AT cost-effectiveness were identified. Two
(Hay & Robin 1991, Alkins & O’Malley 2000) related resource use to expected life gain using USA
registry data. High incremental expected survival of more than seven years in non-smokers resulted
in AT appearing relatively cost-effective. Gildea and colleagues developed a model where health
states were stratified by COPD severity using FEV1-defined ranges. This approach is also adopted in
COPD modelling more broadly. The RAPID trial was powered to detect changes in CT-scanned lung
density. Correspondingly, the patient-level data and model developed by s47G
defined
the
health states by FEV1 predicted and CT lung density decline tracks. This approach is followed in this
assessment. A number of economic models have also been developed to assess the economic
attractiveness and costs of lung transplantation. The two major studies are f
under rom the UK and the
(CTH) Care.
Netherlands. Given that the UK study had larger patient numbers, assumptions for this assessment
are drawn from that study.
1982 Aged
D.3.2.
STRUCTURE OF THE ECONOMIC EVALUATION released
Act and
A cost-utility model based on the decision tree in s47G
was
developed to estimate the expected costs and QALYs associated with AT compared to BSC. The
been
Markov model was developed using Microsoft Excel 2010 and is included as an attachment to this
Health
assessment. There are eight health states i
has n the model, which are defined using FEV1 and CT-scan
lung density decline. The FEV
of
1≥50% and FEV1<50% states were stratified by lung density decline,
Information
categorised as no lung density decline (<0 g/l/year), slow lung density decline (0-2 g/l/year) and
of
rapid lung density decline health state (>2 g/l/year).
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
114
Page 128 of 218
FOI 5155 - Document 4
s45, s47(1)(b)
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
1.
FEV1≥50% predicted, no lung density decline
Department
2.
FEV1≥50% predicted, slow lung density decline
This Freedom
3.
FEV1≥50% predicted, rapid lung density decline
the
4.
FEV1<50% predicted, no lung density decline
by
5.
FEV1<50% predicted, slow lung density decline
6.
FEV1<50% predicted, rapid lung density decline
7.
Lung transplant
8.
Dead
The additional two states of lung transplant and death are also included in the model structure.
Patients were only eligible for lung transplantation at the later stages of disease progression, defined
as FEV1<50% predicted and either slow or rapid decline in lung density. s45, s47(1)(b)
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
115
Page 129 of 218
FOI 5155 - Document 4
s45, s47(1)(b)
The baseline distribution of patients was based on the BSC arm in the RAPID trial. A cohort of 1,000
patients was al ocated to each of the states, and results of the economic evaluation were generated
as cohort-expected value analysis for this population of 1,000. The model’s baseline year was 2019.
All future costs and health benefits are discounted back to this year using a rate of 5%, which is
standard MSAC economic evaluation practice. Higher and lower discount rates are included in a
sensitivity analysis at the conclusion of Section D.
D3.3
ASSUMPTIONS INCORPORATED INTO THE MODEL STRUCTURE
the
Assumptions incorporated into the economic evaluation (summarised in Table 62) relate to the
model’s perspective and type of economic evaluation, along with the sources of evidence, time
horizon, and outcomes used to measure the intervention and comparator.
under
(CTH) Care.
Type of economic evaluation
Given the claim of superiority, a cost-utility economic model has been developed. It is presented as a
1982 Aged
stepped analysis. The first step estimates costs and clinical benefits over the maximum follow-up
released
period for which clinical outcomes have been reported, which is the four-year RAPID trial. As AATD is
Act and
a chronic condition, AT therapy is likely to have longer term costs and benefits. The second step of
the economic modelling approach extrapolates the
been period of analysis for a further 26 years. This
corresponds with an overall time horizon of 30 years, which corresponds to life expectancy at 54
has
Health
years in Australia. Incremental costs and clinical benefits (life years and QALYS) are estimated to
of
calculate ICER.
Information
of
Sources of evidence
The RAPID trial is the key source of clinical evidence, however, the population was limited and the
document
trial was powered to report lung density decline rather than FEV1. Patients in the model are assumed
Department
to transition between states characterised by FEV1 and CT-scan defined lung density. A transition
This Freedom
matrix from individual patients in the RAPID trial was provided by s47G
the
Given that no significan
by t change in FEV1 was measured in RAPID, the proportions falling into each
state in the small RAPID trial are uncertain at the population level. s45, s47(1)(b)
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
116
Page 130 of 218
FOI 5155 - Document 4
Annual transition probabilities were defined for the AT and BSC arms. These parameters were
estimated using results of the RAPID/RAPID OLE trials for the first four years for AT and for the first
two years for BSC. Patients could move between any states as defined by the results of patient
outcome reporting within the RAPID and RAPID-OLE period using patient-level data supplied by CSL
Behring (2017). The rate of CT-scan density decline is a relative measure rather than an absolute
definition of a state. It is likely to stabilise after four years of AT or BSC, therefore patients were
assumed to remain on the same no decline, slow decline or fast decline tracks from year four until
the end of the lifetime projection in the stepped analysis. Patients were assumed to progress from
FEV1≥50% to FEV1<50% based on average patient FEV1 progression in the ADAPT UK registry for BSC,
and an adjusted slower decline for AT patients using the meta-analysis of Chapman et al. (2009).
Patients in the FEV1<50 slow and rapid decline states transition to lung transplantation at a fixed
the
proportion using UK transplantation rates.
To define longer-term survival, parametric models were fitted to patient data from the UK registry,
under
grouped by FEV
Care.
1 and no, slow and rapid decline pathways. The annual probabilities of death for each
(CTH)
health state after the four-year follow-up of the RAPID trial, were based on these models. The
methods used to measure CT lung-density decline in the registry, were noted as being comparable to
1982 Aged
the methods used to measure CT lung density in the RAPID trial. Patients join the year of follow-up
released
of the ADAPT UK registry best matched to cumulative survival on each arm at the end of the RAPID
Act and
trial. Annual rates of mortality for each FEV1/lung density decline state then fol ow the slope of the
parametric model.
been
Patients transitioning from FEV
Health
1>50, to FEV1<50 at later years of follow-up are likely to have
has
overstated annual mortality. This bias is likely to fav
of our the intervention, however, the exact
Information
magnitude of the error is difficult to predict because survival data is not provided by age in the UK
registry dataset. Given that only limited
of numbers of patients transition between FEV>50 and FEV<50
after maximum RAPID fol ow-up, the impact on ICER is likely to be limited. Sensitivity analysis is
document
included at the end of Section D, demonstrating the robustness of the estimated ICER to parametric
model selection. Given high rates of annual mortality for FEV<50/rapid decline, the type of model
This
Department
selected to extrapolate survival for
Freedom this patient group has the largest impact on economic results.
the
Perspective
by
The economic analysis takes the perspective of the Australian health system. Health service costs are
valued at 100% of fee value for MBS items. This perspective is taken by the Federal Government of
Australia and the National Blood Authority (NBA). Budget impact analysis provided in Section E takes
MBS, Pharmaceutical Benefits Advisory Committee (PBAC), state government and private payer
perspectives.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
117
Page 131 of 218
FOI 5155 - Document 4
Time horizon
The base case of the economic evaluation is generated by a trial-period follow-up period using the
four-year RAPID trial. Due to the chronic nature of A1PI deficiency, an extrapolated time horizon
(additional 26 years) was also included to reflect a patient lifetime. The age at baseline was 53.8
years, which aligns with the RAPID study (Chapman et al. 2015).
The model evaluated AT over 30 years to capture all possible differences in costs and outcomes (life
year and QALY) between the modelled cohorts, given that the average life expectancy of Australian
males and females is around 30 years for a 54-year-old. In the sensitivity analysis, the time horizon
was varied to 20 years and 40 years. Given that the median survival time from diagnosis as a severe
A1PI deficient patient treated with BSC is less than 10 years (Tanash et al. 2010), sensitivity analyses
the
on time horizons at 30 years and 40 years have limited impact on the estimated ICER.
Outcomes
under Care.
QoL, general y assessed by EQ-5D, is the main outcome of cost-utility analysis. Measure
(CTH) s of QoL that
could be transformed into utility were not reported in the RAPID study. CSL Behring (2017)
presented EQ-5D values stratified by FEV1% predicted from the UK ADAPT registry using data from
1982 Aged
244 patients not receiving maintenance therapy (Ejiofor and Stockley, 2015). A weighted average of
released
the utilities in patients with a FEV
Act
1% <30, 30-35, 35-40, 40-45 and 45-50 was calculated to derive the
and
utility for the FEV1 <50% predicted group health state. The utility value for FEV1 >50% was taken
been
from the dataset. These values are used in this assessment. Lung transplant utility values were taken
from the UK. Results reported by Groen et al. (2004) in The Netherlands and Anyanwu et al. (2001)
has
Health
in the UK indicate that utility increases substantially after transplantation. The data from Anyanwu
of
et al. (2001) are used in the base case, as Groen et al. (2004)
Information reported a smaller transplant patient
population.
of
Methods used to generate results
document
The economic model used to generate the results is an expected value cohort analysis for 1,000
Department
patients. A Markov
This model was developed, through which patients transition between each state
Freedom
based on annual transition probabilities. As indicated, these were defined for the first four years of
the
AT using RAPID/RAPID OLE and for the first two years of BSC using RAPID. Patients are assumed to
by
remain on the no, slow or rapid decline track for the extrapolated lifetime analysis. Patients move
between FEV1>50 and FEV1<50 based on the average annual FEV1 decline rate observed in the UK
AATD registry. Transitions to mortality are defined by parametric survival models for each health
state. A fixed fraction of FEV1<50 slow and rapid decline patients are assumed to receive
transplantation. The simulation began with the hypothetical cohort of 1,000 patients in one of the
six FEV1% predicted/density decline health states (according to the distribution of patients from the
BSC arm of the RAPID trial), and ran for four years (trial) or 30 years (lifetime). The evaluation then
compares the expected costs and clinical outcomes (life years, QALYs) between the treatment
options.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
118
Page 132 of 218
FOI 5155 - Document 4
Health states
The model includes eight states based on FEV and CT scan-measured lung density decline (presented
in Table 69). Death is a state from which patients could not transition, and lung transplant patients
could only transition to death. Patients could not transition between the no decline, slow decline
and rapid decline health states after four years, but they could progress from FEV1≥50% to FEV1<50%
based on a background rate of decline for BSC and adjusted slower progression for AT.
Table 69
Economic model health states
Health state
Description
Possible transitions to other health states
FEV1≥50% predicted, no lung
density decline
FEV
the
1≥50% predicted, slow lung
density decline
No lung density decline is Patients could not transition between the no decline,
FEV1≥50% predicted, rapid lung
defined as <0 g/l/year, slow slow decline, but rapid decline health states after 4
density decline
lung density decline as 0-2 years and could simultaneously progress from
under
FEV
g/l/year and rapid lung density FEV1≥50% to FEV1<50%. Parametric models are
Care.
1<50% predicted, no lung
(CTH)
density decline
decline as >2 g/l/year
used to extrapolate annual mortality after 4 years.
RAPID trial data is used in the trial period.
FEV1<50% predicted, slow lung
density decline
1982 Aged
FEV1<50% predicted, rapid lung
released
density decline
Act and
A fraction of FEV1<50%
Lung transplant
predicted, rapid and slow lung
density decline transition to
Lung transplant, Death
been
this state.
The proportion of the cohort
has
Health
that die from any cause, even
of
those that are unrelated to
Information
morbidity, transition into this
None
Death
health state.
of
(this is an absorbing health state)
No costs or benefits are
accrued i
document n this health state.
Abbreviations:
FEV1 = Forced expired volume in 1 second.
Department
Cycle length
This Freedom
the
The economic model employs a cycle length of one year. This step was chosen as s47G
provided AT and BSC pa
by tient-transition data on an annual basis, along with parametric model for
survival derived from the UK registry.
Discount rate
Costs and clinical benefits (difference in QALYs between AT and BSC) are discounted at 5% per
annum. The impact of discounting is explored in sensitivity analyses. A half-cycle correction is
applied.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
119
Page 133 of 218
FOI 5155 - Document 4
Comparator
The main comparator for AT is BSC, described as a range of interventions including pharmacological
(e.g. bronchodilators, systemic corticosteroids), non-pharmacological (e.g. oxygen therapy) and
preventative measures such as vaccinations.
D.4.
INPUTS TO THE ECONOMIC EVALUATION
The following sections summarise the clinical and economic input parameters included in the
economic evaluation.
D.4.1.
CLINICAL INPUT PARAMETERS
the
Baseline patient demographics
Table 70 summarises baseline patient characteristics of the modelled patient population. Age affects
under
the modelling time frame, given that a patient with a base age of 54 has a life exp
(CTH) ectancy
Care. of 30
years. This variable does not affect the treatment effectiveness or natural history of the disease in
the model. The distribution among health states at commencement was taken from the patient
1982 Aged
profile in the RAPID trial.
released
Act and
Table 70
Baseline patient and disease characteristics of the modelled patient cohort
Parameter
Input
Source
been
Age of population at baseline
53 years
RAPID
Health
FEV1 and lung sensitivity decline state at baseline
has
FEV
of
1>50 no decline (%)
7%
RAPID
FEV
Information
1>50 slow decline (%)
13%
RAPID
FEV
of
1>50 rapid decline (%)
20%
RAPID
FEV1<50 no decline (%)
8%
RAPID
FEV
document
1<50 slow decline (%)
34%
RAPID
FEV1<50 rapid decline (%)
18%
RAPID
Abbreviations: FEV
Department
1 = Forced expired volume in 1 second.
This
Freedom
As described in Section D.2 above, the model is built to perform a Markov process, so patient
the
transition into different states over the course of the model projection period. There were only eight
by
Australian patients the RAPID multicentre global trial, so there is uncertainty about the proportions
of Australian populations allocated to each health state. These proportions are varied in a sensitivity
analysis, with all patients being assumed to commence as FEV1>50, and all FEV1<50 equally
distributed among lung density decline groups. Changing the baseline patient distribution only has a
smal impact on the estimated ICER.
Transition probabilities – effectiveness of AT and BSC as captured in the model
Transition probabilities are derived from RAPID IPD, provided by CSL Behring (2017). Patients move
between FEV1<50 and >50 CT-scan lung density decline states according to that observed over four
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
120
Page 134 of 218
FOI 5155 - Document 4
years for AT (RAPID and RAPID OLE), and two years for BSC (RAPID). Annual probabilities are
provided in Table 71. A constant proportion of FEV1<50 slow and rapid CT-scan lung decline patients
are assumed to receive lung transplantation.
s47(1)(b)
the
under
(CTH) Care.
Aged
released 1982
Act and
been
After four years, patients can only transition between FEV1>50 and FEV1<50 states that match their
has
Health
no decline, slow decline and rapid decline pathway. Patients cannot revert back to FEV1>50, once
of
they have transitioned to FEV1<50. The baseline rate of FEV1 decline was derived from the UK
Information
registry and was assumed to be the same for all FEV1>50 states.
of
s45, s47(1)(b)
document
This Freedom
Department
the
by
Table 72
Health state transition probabilities – Years >4 years
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
121
Page 135 of 218
FOI 5155 - Document 4
FEV1>50
FEV1>50
FEV1>50
FEV1<50 no
FEV1<50
FEV1<50
no decline
slow
rapid
decline
slow
rapid
decline
decline
decline
decline
BSC
s45, s47(1)(b)
AT
s45, s47(1)(b)
the
under
(CTH) Care.
Abbreviations:
AT = augmentation therapy,
BSC = best supportive care,
FEV1 = Forced expired volume in 1 second.
1982 Aged
Trial mortality
released
Annual mortality probabilities are trial mortalities taken from four years of RAPID/RAPID OLE for AT,
Act and
and two years for BSC from RAPID - s47G
Death is assumed
been
to be the same for all patients in each arm of the model regardless of health state. Annual rates are
provided in Table 73.
has
Health
of
Table 73
Health state dispositions at month 24 and month 30 and associated transition probabilities – patients
with severe depression at baseline
Information
of
Annual probability of death
Cumulative survival
AT
BSC
AT
BSC
document
Year 1
1.08%
2.30%
98.92%
97.70%
Year 2
0.00%
1.18%
98.92%
96.55%
Department
Year 3
0.71%
98.22%
This
Freedom
Year 4
0.00%
98.22%
the
Abbreviations:
AT = augmentation therapy,
BSC = best supportive care,
FEV1 = Forced expired volume in 1 second.
by
Extrapolation of mortality after the trial period
As outlined in Section C, a series of parametric survival models provided by s47G
are
used to model survival (Figures 21, 22 & 23). Results of the extrapolations are presented as a series
of Markov traces for the AT and BSC arms, along with a trace showing the difference in patient
numbers by state as a result of AT delivery. For both AT and BSC it is evident that large numbers of
patients transition out of FEV1>50 rapid decline and FEV1<50 slow decline in the early years of the
model projection. The key difference between AT and BSC, is that larger numbers of patients are
retained in the FEV1<50 slow decline state compared to the FEV1<50 rapid decline state, as a result
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
122
Page 136 of 218
FOI 5155 - Document 4
of AT. The effective annual rate of death across the AT arm is less than that of BSC, resulting in an
increase of three life years for an average patient.
s45, s47(1)(b)
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
123
Page 137 of 218
FOI 5155 - Document 4
s45, s47(1)(b)
the
under
(CTH) Care.
Aged
released 1982
Act and
D.4.2.
ECONOMIC INPUT PARAMETERS been
AT is expected to change resource use in the healthcare system due to cost of the product and
Health
change in costs associated with ongoing dise
has ase management (Table 74).
of
Cost of Intervention
Information
of
AT product and delivery services
document
AT delivery involves costs of the product and medical services. These costs are provided in Table 74.
Department
Table 74
Resources associated with AT and disease management costs by COPD severity
This Freedom
Price per
Number of Proportion
the
Total
MBS Item
Provider of unit of
vials or
availing
resource
resource
services
service /
annual
Source
by
(AU$) per year product (%) cost (AU$)
AT product cost
and delivery
s45, s47(1)(b)
Based on 60mg/kg,
1,000ml vial, adherence
94% and weight 76 kg
Costs of infusion
MBS
65.05
52.00
1.00
3,382.60
MBS item, 13915
Subtotal
s45, s47(1)
(b)
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
124
Page 138 of 218
FOI 5155 - Document 4
Mild-Moderate COPD >FEV1 50
GP consultations
General
Practioner
37.60
2.33
1.00
87.61
MBS item 23, Level B
using Thomas et al. 2014
Weighted Average costs
Hospital Services
Hospital
7,017.54
1.00
0.04
280.70
for DRG items E65A/E65B,
using Thomas et al. 2014
for frequency
Subtotal
368.31
Severe COPD
GP consultations
General
Practioner
37.60
3.33
1.00
125.21
MBS item 23, Level B
using Thomas et al. 2014
Weighted Average costs
Hospital Services
Hospital
7,017.54
1.00
0.10
701.75
for DRG items E65A/E65B,
using Thomas et al. 2014
the
for frequency
Subtotal
826.96
Very Severe COPD
under Care.
GP consultations
General
(CTH)
Practioner
37.60
3.67
1.00
137.99
MBS item 23, Level B
using Thomas et al. 2014
Weighted Average costs
Hospital Services
Hospital
7,017.54
1.00
0.16
1,122.81
for DRG items E65A/E65B,
1982 Aged
using Thomas et al. 2014
for frequency
released
Subtotal
Act
1,260.80
and Thomas et al. 2014 -
Weighted Severe
assuming 74% of patients
been
COPD (FEV
74%
939.76
1<50)
had severe COPD and 26%
had very severe COPD
has
Health
Lung transplant costs
of
Lung transplant
Hospital
AR-DRG A03Z, NHCDC
Information
first year
and MBS
122,332.97
1.00
1.00
153,159.28 round 18 adjusted by AIHW
health price index.
of
Lung transplant
Hospital
follow-up years
and MBS
13,837.00
1.00
1.00
14,542.69
Anyanwu 2002 - adjusted
by AIHW health price index
document
Abbreviations:
AIHW = Australian Institute of Health and Welfare,
AR-DRG = Australian Refined Diagnosis Related Groups,
AT =
augmentation therapy,
COPD = chronic obstructive pulmonary disease,
DRG = diagnosis related groups,
FEV1 = forced expiratory volume
in 1 second,
GP = general practitioner,
MBS = Medicare benefits schedule.
Department
This Freedom
COPD Medical Services Costs
the
The costs of COPD medi
by cal services for disease management are taken from the frequencies of GP
and hospitalisations by COPD severity stage reported in a UK survey by Thomas et al. (2014). They
are indexed to 2018 using the AIHW health price index.9
9 The AIHW price index is reported until 2016 (in July 2018). The value in this year of 1.7 is also used for 2017 and 2018
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
125
Page 139 of 218
FOI 5155 - Document 4
Utility values
The derivation of utilities was outlined in Section C using UK registry data (Ejiofor & Stockley 2015)
provided by CSL Behring (2017). UK registry values are included in the economic model (Table 75).
s45, s47(1)(b)
the
under
(CTH) Care.
Of note, the trial-estimated utilities for follow-up lung transplant appear to be higher than those
reported from the Australian population. For example, event-free values of 0.80 for 75+ years were
1982 Aged
found as part of the Queensland 2011 Self-Reported Health Status survey (Clemens et al. 2014), and
released
a value of 0.70 for 71+ years in the short form 6D (SF-6D) as part of the Household, Income and
Act and
Labour Dynamics in Australia (HILDA) survey (Norman et al. 2013). Given these uncertainties they
are subjected to a range of sensitivity analyses presen
been ted at the end of Section D.
D.5.
R
Health
ESULTS OF THE ECONOMIC E
has
VALUATION
of
D.5.1.
HEALTH CARE COSTS BY RESOURCE TYPE
Information
The costs per patient for AT and BSC for t
of he trial period analysis and the stepped lifetime horizon are
presented in Table 76. Costs presented are averages generated by the model. It is evident that the
document
cost of the AT product and its delivery are the dominant costs for AT, and resources associated with
COPD management are minor. Lung transplant costs are small compared to AT product and delivery
This
Department
costs. Costs are greater for the AT a
Freedom rm as AT patients have higher survival than do BSC patients.
the
Table 76
Health care costs by resource type for base-case analysis (1,000-person cohort)
by
% Total
AT (AU$)
BSC (AU$)
Incremental Cost
(AU$)
Incremental
Cost
Trial period
Undiscounted
Undiscounted
Undiscounted
Undiscounted
s45, s47(1)(b)
Mild-Moderate COPD >FEV1 50
635,831
580,570
55,260
0%
Severe COPD <FEV1 50
2,747,065
2,635,731
111,334
0%
Lung transplant
17,480,957
17,430,173
50,784
0%
Total
s45, s47(1)(b)
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
126
Page 140 of 218
FOI 5155 - Document 4
% Total
AT (AU$)
BSC (AU$)
Incremental Cost
(AU$)
Incremental
Cost
Lifetime
Undiscounted
Undiscounted
Undiscounted
Undiscounted
s45, s47(1)(b)
Mild-Moderate COPD >FEV1 50
1,227,205
881,163
346,042
0%
Severe COPD <FEV1 50
5,460,430
4,013,603
1,446,827
0%
Lung transplant
61,086,623
46,950,532
14,136,091
1%
s45, s47(1)(b)
Abbreviations:
AT = augmentation therapy,
BSC = best supportive care,
COPD = Chronic Obstructive Pulmonary Disease,
FEV1 =
Forced expired volume in 1 second.
the
D.5.2.
HEALTH OUTCOMES PER PATIENT BY STEP AND BY HEALTH STATE
Table 77 presents the average outcomes (per patient) generated by the economic model for LY or
under
QALY in the trial follow-up period. It is evident that most incremental LYs and QALYs accrue t
Care. o the
(CTH)
FEV<50 slow decline state for AT.
Table 77
Average patient health outcomes by health state and by outcome measu
1982
re for trial an
Aged
alysis
released
AT
BSC
Incremental
% Total
Act
and
Incremental
Trial period
been
# LYGs
Undiscounted
Undiscounted
Undiscounted
Undiscounted
FEV1>50 no decline
0.1
0.0
0.1
33%
has
Health
FEV1>50 slow decline
1.3
1.2
0.1
48%
of
FEV1>50 rapid decline
0.3
0.4
-0.1
-27%
Information
FEV1<50 no decline
0.3
0.1
0.2
62%
of
FEV1<50 slow decline
2.1
1.3
0.8
299%
FEV1<50 rapid decline
0.5
1.4
-0.9
-313%
document
Lung transplant - first year
0.1
0.1
0.0
0%
Lung transplant - following years
0.2
0.2
0.0
0%
Department
Total
This
4.9
4.6
0.3
100%
Freedom
# QALYs
Undiscounted
Undiscounted
Undiscounted
Undiscounted
the
FEV1>50 no decline
0.1
0.0
0.1
37%
by
FEV1>50 slow decline
1.0
0.9
0.1
54%
FEV1>50 rapid decline
0.2
0.3
-0.1
-31%
FEV1<50 no decline
0.2
0.0
0.1
52%
FEV1<50 slow decline
1.3
0.8
0.5
254%
FEV1<50 rapid decline
0.3
0.8
-0.5
-266%
Lung transplant - first year
0.1
0.1
0.0
0%
Lung transplant - following years
0.1
0.1
0.0
0%
Total
3.3
3.1
0.2
100%
Abbreviations:
AT = augmentation therapy,
BSC = best supportive care,
FEV1 = Forced expired volume in 1 second,
LYGs = life years
gained,
QALY = quality-adjusted life year.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
127
Page 141 of 218
FOI 5155 - Document 4
Table 78 presents the average outcomes (per patient) generated by the economic model for LY or
QALY in the lifetime period. Again, it is evident that most incremental LYs and QALYs accrue to the
FEV<50 slow decline state for AT.
Table 78
Health outcomes by health state and by outcome measure for lifetime analysis (Per patient)
Lifetime
AT
BSC
Incremental
% Total Incremental
# LYGs
Undiscounted
Undiscounted
Undiscounted
Undiscounted
FEV1>50 no decline
0.2
0.0
0.2
6%
FEV1>50 slow decline
2.6
1.8
0.8
27%
FEV1>50 rapid decline
0.5
0.5
0.0
-1%
FEV1<50 no decline
0.5
0.1
0.4
14%
FEV1<50 slow decline
4.4
2.0
2.3
79%
FEV
the
1<50 rapid decline
0.9
2.2
-1.2
-41%
Lung transplant - first year
0.2
0.2
0.0
2%
Lung transplant - following years
2.0
1.5
0.4
15%
Total
11.3
8.4
3.0
100%
under Care.
# QALYs
Undiscounted
Undiscounted
Undiscounted
U
(CTH)
ndiscounted
FEV1>50 no decline
0.2
0.0
0.1
7%
FEV1>50 slow decline
2.1
1.4
0.6
31%
FEV
1982 Aged
1>50 rapid decline
0.4
0.4
0.0
-1%
FEV
released
1<50 no decline
0.3
0.1
0.2
12%
FEV
Act and
1<50 slow decline
2.6
1.2
1.4
68%
FEV1<50 rapid decline
0.6
1.3
-0.7
-36%
Lung transplant - first year
0.2 been 0.1
0.0
2%
Lung transplant - following years
1.5
1.2
0.3
17%
Health
Total
7.8
5.7
2.0
100%
has
Abbreviations:
AT = augmentation therapy,
BSC = best supportive care,
FEV
of
1 = Forced expired volume in 1 second,
LYGs = life years
gained,
QALY = quality-adjusted life year.
Information
D.5.3.
INCREMENTAL COSTS AND EF
of
FECTIVENESS
The incremental cost and the incremental effectiveness of AT for an average patient are presented
document
in Table 79 for the 1000-person cohort. The ICER is presented as the incremental cost of achieving an
additional QALY. It is evident that the life time ICER
Department is s47(1)(b) per QALY and for the trial period is
This Freedom
s47(1)(b)
.
the
Table 79
Incremental Cost Effectiveness Ratio (1,000-patient cohort)
by
Cost (AU$)
Incremental Effectiveness Incremental
ICER
cost (AU$)
(QALYs)
effectiveness
Trial period
A1PI Augmentation Therapy
s45, s47(1)(b)
Best Supportive Care
18,531,803
2,822.6
Lifetime
A1PI Augmentation Therapy
s45, s47(1)(b)
Best Supportive Care
37,389,939
4,525.4
Abbreviations:
A1PI = Aplha-1 proteinase inhibitor;
ICER = incremental cost-ef ectiveness ratio;
QALY = quality-adjusted life year.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
128
Page 142 of 218
FOI 5155 - Document 4
D.6.
SENSITIVITY ANALYSES
A trial period (4 years) and lifetime extrapolation (30 years) are included in the economic analysis.
Only limited incremental clinical benefits accrue during the trial period, as patients transition to
health states during this period. Mortality differences become more evident between the AT and
BSC arms over the following five to 20 years of the lifetime projection. Thus, the ICER for the trial
period is far greater (i.e. less cost effective) than that of the lifetime projection. Sensitivity analyses
are presented for the lifetime analysis using the following univariate changes and scenario analyses
(Table 80):
•
The average age of entry in the baseline is 53 years (based on RAPID trial participants). A
lifetime projection of 30 years is used in the economic model, based on average life
expectancy for males and females in Australia. This is decreased to 2
the 0 years and
increased to 40 years to gauge model results over two lengths of maximum follow-up. It is
evident that the ICER is relatively insensitive to this assumption, as most mortality occurs
under
within the first 20 years of the modelling projection.
(CTH) Care.
•
The baseline distribution of patients across FEV1 and CT lung-density decline states was
based on the RAPID trial. It is assumed that FEV
1982 Aged
1>50 no decline accounts for 7% of the
starting patient population, FEV
released
1>50 slow decline 13%, FEV1>50 rapid decline 20%,
Act and
FEV1<50 no decline 8%, FEV1<50 slow decline 34%, and FEV1<50 rapid decline 18%. The
RAPID trial was conducted across multiple countries with only eight patients recruited in
been
Australia. There is considerable uncertainty about the characteristics of patients who
would be recruited, as limited public data is available abo
Health ut this population in relation to
has
FEV
of
1 and CT lung-density decline status. Two sensitivity analyses are undertaken. The first,
Information
where all patients are assumed to be FEV1>50 and equal y distributed among CT lung-
density no decline, slow and r
of apid decline; and the second, with al patients FEV1<50 and
equal y distributed among CT lung-density no decline, slow and rapid decline. It is evident
document
that baseline characteristics only have limited impact on the ICER as patients transition
into FEV1<50 within a few years.
This Freedom
Department
•
An average weight of 75.9kg is used in the model (based on RAPID trial participants). This
the
weight is in line with average adult weights in Australia as reported by ABS and a range of
by
other cited trials. Weight is varied to assess ICER impact. The lower bound weight of 68 kg
is not sufficient to change the number of vials needed per week, as part-vials are rounded
to a whole number (i.e. five vials based on 60mg dosing). The upper weight of 83kg results
in six vials required per week. This has a large impact on the estimated ICER as the cost of
the AT product is the key driver of the model.
• Lung transplant probability (4.17%) and probability of death resulting from lung transplant
(8.53%) are derived from UK data for the base model. Only patients with FEV1<50 and in
slow and rapid decline tracks are assumed to be eligible for lung transplantation. Changes
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
129
Page 143 of 218
FOI 5155 - Document 4
in these variables have a limited impact on the estimated ICER, as only a small proportion
of patients from severe states are assumed to receive a transplant.
• A base discount rate of 5% was used. Higher rates of 7.5% and lower rates of 2.5% are also
included. Changes in discount rate have an impact because much of the clinical benefit
occurs between years five and 20 of the model projection, where discounting has a large
effect on present value. The stepped analysis shows that only a small proportion of
avoided mortality and morbidity accrues within the trial period. For example, only 9% of
incremental life years gained occur in the four-year trial period, and a similar percentage
is estimated for QALYs.
• The rate of FEV
the
1 decline between BSC and AT patients differed in the meta-analysis
conducted by Chapman et al. (2015). FEV1 decline was estimated to reduce by 26% as a
result of AT usage, and this assumption is employed in the model. The base model
under
assumes that patients transition to no, slow and rapid decline pathways during the trial
(CTH) Care.
period, then follow these tracks over a lifetime. This assumption is changed in the
sensitivity scenario “transitions within RAPID continue for lifetime”. This scenario allows
patients to keep transitioning between states for 30 years as th
1982 ey did i
Aged n the four-year
released
RAPID trial period. The estimated ICER from allowing lifetime transition between decline
Act and
states does not vary substantially because most patients have moved to severe states
within the four years of the trial period, then follow defined survival curves for each
been
health state.
has
Health
• Annual patient mortality is taken from the AT and BSC arms of the RAPID trial and applied
of
to al BSC and AT health states across the four ye
Information ars of AT follow-up and two years of BSC
follow-up. After this period, m
of ortality is estimated using parametric models fitted to UK
registry data for non-lung transplant FEV1/CT density decline states. Under the base
modelling assumption, pa
document tients hinge to the UK survival curves based on year of best
match, rather than year of follow-up. As age is not specified in the survival data, the
Department
relative ris
This k of mortality by age and disease state is uncertain. If annual patient mortality
Freedom
by year from the UK registry is applied to the AT and BSC arms following the respective
the
years of maximum follow-up (rather than hinging to year of best fit), then the estimated
by
ICER changes considerably. The ICER becomes less cost-effective.
•
UK survival data is extrapolated using a range of parametric models fitted using clinical
plausibility and AIC criteria provided by CSL Behring (2017). In most cases the Gompertz
model is the best fit, hence this model is used across all non-transplant states. The model
is varied in sensitivity analyses, which included use of the Log-logistic, Lognormal, Weibull,
Exponential and Generalised Gamma specifications. For the FEV1 >50 and FEV1 <50 slow
decline states, changes in the model lead to an increase in the estimated ICER. The
opposite occurred for FEV1 <50 no decline and FEV1 <50 rapid decline states. The choice of
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
130
Page 144 of 218
FOI 5155 - Document 4
model for the FEV1<50 rapid decline state had the largest impact on the estimated ICER.
The use of Lognormal, Generalised Gamma and Weibull models results in the ICER being
10% more cost-effective, while use of the Exponential model resulted in a 10% decrease in
cost-effectiveness. Large numbers of patients transition to this state during the trial
period, particularly on the BSC arm. Assumptions about annual mortality correspondingly
have a large impact on the estimated ICER.
•
s47(1)(b)
. Changes in the
number of vials used or product price is the key driver of cost effectiveness. The base cost
of AT assumes a price per 1,000 ml s47(1) This varies from a low value of s47(1 per
(b)
)(b)
1,000ml vial to a high value of s47(1 per 1,000ml vial. The estimated ICER varies
)(b)
the
considerably between s47(1)(b) and s47(1)(b) . The MBS cost of AT delivery is also varied
by 20% from the base cost per infusion of s47(1) This has limited impact on cost-
(b)
effectiveness results.
under
(CTH) Care.
•
In many reviewed COPD economic models, disease management costs were an aggregate
of maintenance and acute care costs during flare ups. The frequency of flare ups was not
explicitly modelled in this assessment. RAPID trial results s
1982 howed a n
Aged on-significant
difference between these occurrences on the BSC and A
released T arms. As in the s47G
Act and
model, Thomas and colleague’s (2014) collation of COPD costs by GOLD stages was
used to estimate disease management costs for health states in the economic model. The
been
Thomas et al. (2014) analysis included acute care proportions for each state. Proportion
hospitalised for mild COPD was 4%, for severe COPD 10%
Health , and for very severe COPD 16%.
has
These proportions are uncertain for AT. They
of are varied by 20% for each COPD state. This
Information
variation has limited impact as economic results are governed by AT product costs. The
proportion of severe COPD p
of atients who are very severe, assumed to be 74% in the base
case, is also varied. Similarly, this scenario had limited impact on the estimated ICER.
document
•
The base cost for lung transplant is estimated to be $153,159, using Australian AR-DRG
Department
costs we
This ighted by separations. There is uncertainty about how much this procedure may
Freedom
cost and it is varied in a univariate change of 20%. Given the small number of patients who
the
receive transplantation in the model, and the cost of the procedure relative to AT product
by
costs, this variation has limited impact. Lung transplant follow-up costs of $14,543 per
year are also varied by the same magnitude and ICER results do not change significantly.
•
Utilities are specified for the patient group over FEV1 >50 (0.79) and FEV1 <50 (0.59). There
is uncertainty around these estimates, as no account for lung-decline status is included. A
sensitivity analysis is included where these values are changed by 5%. It is evident that the
ICER does not vary considerably. This is largely because much of the LY and QALY benefits
are derived from an increase in the years of life lived. Lung transplant first year and
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
131
Page 145 of 218
FOI 5155 - Document 4
following year utilities are also varied. Given the small relative patient number, this
scenario has a limited impact on the estimated ICER.
Table 80
Sensitivity analysis for lifetime analysis
Parameter
Analysis
Incremental cost
Incremental
ICER
effect
Base Case
s47(1)(b)
1,301
s47(1)(b)
Background assumptions
Years of follow-up (30 years)
20
1,274
40
1,308
RAPID baseline distribution by
All FEV1>50
1,511
FEV1
All FEV1<50
1,182 the
Average weight (75.9kg)
68
1,301
83
1,301
Discount rate (5%)
7.5%
1,065
under
2.5%
1,613
(CTH) Care.
Lung transplant probability
2.1%
1,305
(4.17%)
6.3%
1,287
Probability of death - lung
4.3%
1,
1982 369
Aged
transplant (8.53%)
12.8%
released 1,255
Act
Parameter
Analysis
Incremental cost
Increm
and
ental
ICER
effect
A1PI Augmentation Therapy
been
delivery costs
s47(1)(b)
s47(1)(b)
Health 1,301
s47(1)(b)
has
1,301
of
Cost per infusion ($65)
Information
1,301
1,301
of
Disease management costs
Mild COPD proportion
3.2%
s47(1)(b)
1,301
s47(1)(b)
document
hospitalised (4%)
4.8%
1,301
Severe COPD proportion
8.0%
Department
1,301
This
hospitalised (10%) Freedom 12.0%
1,301
the
Very severe COPD proportion
12.8%
1,301
hospitalised (16%)
19.2%
1,301
by
Proportion of severe COPD
59.2%
1,301
patients very severe (74%)
88.8%
1,301
Lung transplant costs
$122,527
1,301
($153,159)
$183,791
1,301
Lung transplant follow-up costs
$11,634
1,301
($14,543)
$17,451
1,301
Effects
FEV1 >50 survival model
Log-logistic
1,281
(Gompertz)
Lognormal
1,292
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
132
Page 146 of 218
FOI 5155 - Document 4
s47(1)(b)
Weibull
s47(1)(b)
1,283
Exponential
1,308
Generalised
1,287
Gamma
FEV1 <50 no decline survival
Log-logistic
1,330
model (Gompertz)
Lognormal
1,327
Weibull
1,310
Exponential
1,417
Generalised
1,326
Gamma
FEV1 <50 slow decline survival
Log-logistic
1,224
model (Gompertz)
Lognormal
1,190
Weibull
1,211 the
Exponential
1,236
Generalised
1,191
Gamma
under Care.
FEV
(CTH)
1 <50 rapid decline survival
Log-logistic
1,411
model (Gompertz)
Lognormal
1,462
Weibull
1,452
1982 Aged
Exponential
1,177
released
Generalised
1,468
Act
Gamma
and
Survival model hinged to last
Year of
480
been
year of rapid through best fit
maximum
follow-up of
each arm
has
Health
Patients follow same lung
Transitions
1,290
of
density decline after 4 years
within RAPID
Information
continue for
lifetime
of
Utilities
FEV1 >50 (0.79)
0.83
1,326
document 0.75
1,276
FEV
Department
1 <50 (0.59)
0.62
1,332
This Freedom 0.56
1,271
the
Lung transplant – first year
0.78
1,302
(0.74)
0.70
1,300
by
Lung transplant - following year
0.81
1,309
(0.77)
0.73
1,293
Abbreviations:
A1PI = alpha-1 proteinase inhibitor;
AT = augmentation therapy,
COPD = chronic obstructive pulmonary disease;
FEV1 =
forced expiratory volume in 1 second;
ICER = incremental cost-effectiveness ratio;
QALY = quality-adjusted life year.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
133
Page 147 of 218
FOI 5155 - Document 4
Key results from the sensitivity analysis are summarised in Table 81.
Table 81
Key drivers of the economic model
Description
Method/Value
Impact
The average dosing for AT is taken from the
The base cost of AT assumes a price per
RAPID trial and applied to an average weight of 1,000 ml s47(1)(b) This is varied by low and
Cost of the AT product
75.9 kg. The number of vials (rounded to a whole high values of s47(1)
(b)
to s47(1)
(b)
per 1,000ml vial.
number) is multiplied by average, high and low The estimated ICER varies considerably
AT product prices
between s47(1)(b) and s47(1)(b) per QALY.
There were considerable dif erences in transition A higher number of patients move to the
Transition between FEV
FEV1<50 decline states on the BSC arm in
1 between health states for the AT and BSC arms
and CT density decline
in the RAPID trials. The economic model
RAPID. Movement during the trial period
during RAPID drives
assumes movement to no, slow and rapid decline drives economic results. Al owing transition
clinical benefit
tracks during the trial period is sustained for a
between no, slow and rapid tracks after 4
the
lifetime.
years has limited impact on the estimated
ICER.
In most cases the Gompertz model is the best fit
model to extrapolate survival and this model is
The specification of the FEV<50 rapid decline
model had the largest impact on the
under
Selection of extrapolation used across all non-transplant states. The model estimated ICER. The use of Lognormal
Care. ,
(CTH)
model for the FEV
is varied as part of sensitivity analyses which
1<50
Generalised Gamma and Weibull models
rapid decline group
included use of the Log-logistic, Lognormal,
results in the ICER being 10% more cost-
survival
Weibull, Exponential and Generalised Gamma
specifications. Large numbers of patients
effective, while use of the Exponential model
1982 Aged
transition to this state during the trial period,
resulted in a 10% decrease in cost-
particularly on the BSC arm.
effectiveness.
released
Act
Disease management costs in many reviewed
and
COPD economic models were an aggregate of This variation has limited impact as economic
maintenance and acute-care costs during flare results are governed by AT product costs.
been
Disease management
ups. The frequency of flare ups was not explicitly The proportion of severe COPD patients who
costs for COPD
modelled in this assessment. The Thomas et al. are very severe, which is assumed to be 74%
Health
2014 analysis included ac
has ute-care proportions for in the base cases is also varied. Similarly,
each state. They are varied by 20% for each
this scenario had limited impact on the
of
COPD state
estimated ICER
Information
Abbreviations:
AT = augmentation therapy,
BSC = best supportive care,
COPD = chronic obstructive pulmonary disease,
CT =
computed tomography,
FEV
of
1 = forced expiratory volume in 1 second,
ICER, incremental cost-effectiveness ratio;
QALY, quality-adjusted
life year.
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
134
Page 148 of 218
FOI 5155 - Document 4
SECTION E
FINANCIAL IMPLICATIONS
E.1.
JUSTIFICATION OF THE SELECTION OF SOURCES OF DATA
Section E presents the financial budget impact for the potential listing of AT using an epidemiological
approach over a six-year period, based on an estimation of the number of patients eligible for
treatment. A1PI deficiency is associated with COPD, however, emphysema is typically the main
manifestation. Estimating the eligible population for AT has been calculated from the estimated
proportion of COPD patients in Australia, combined with an estimated prevalence of ZZ phenotypes
and rate of diagnosis.
the
The data sources used to estimate the number of patients potential y eligible for treatment with AT
are provided in Table 82. Toelle et al. (2013) estimated the prevalence of COPD in Australia between
under Care.
2006 and 2010. The authors undertook a cross-sectional random survey of adults 40 ye
(CTH) ars and older
across six purposely selected diverse sites. Interviewees answered a standardised questionnaire and
performed FEV
1982 Aged
1 and FVC tests. The prevalence of GOLD Stage II or higher COPD was estimated at
released
7.5% among the 1,620 surveyed men and 1,737 women. Act and
The prevalence of COPD among Australians aged 45 years and older was also reported by the
been
Australian Institute of Health and Welfare as part of Australian Bureau of Statistics National Health
Surveys in 2014–15. Surveys indicated that 5.2% of Australians aged 18 years self-reported a
has
Health
diagnosis of COPD, chronic bronchitis or emphysema (AIHW 2018). Age-stratified prevalence varied
of
from 2.5% in 45- to 54-year-old males, to 10% in those aged 75 years or older. The 45- to 54-year-old
Information
rate was similar in females, but only 8.1% in those aged 75 years or older. COPD hospitalisations per
of
100,000 population in 2015–16 were estimated to be 804.7 in males and 666.6 for females.
Differences between the Toelle et a
document l. (2013) and AIHW (2018) estimates could be associated with
differences in response rate, age groupings and methods of diagnosis.
This Freedom
Department
Using ABS Australian population statistics, the estimated national proportion of 40- to 65-year olds,
the
and an average population growth rate of 1.6% per year, it is estimated that this age group will
account for around eight
by million Australians in 2019. The numbers suffering from COPD (GOLD Stage
I or higher) is estimated using the Toelle et al. (2013) study prevalence, along with a lower
prevalence estimate (6.8%) being included in a sensitivity analysis.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
135
Page 149 of 218
FOI 5155 - Document 4
Table 82
Summary of the key assumptions used in the financial impact assessment
Assumption
Base case
Sensitivity Reference
analysis
Population and incidence
Australian population
24,770,700
Australian population in December 2017 quarter. ABS
(2018). The 2017 estimate is inflated by the growth
rate of 1.6% for the 2019 base year.
Australian population aged 40-65
7,872,729
13,215,161 The proportion of the Australian population aged 40 to
years
65 years is used for prevalence estimate given life
expectancy for AATD. A higher population estimate is
included in a sensitivity analysis based on the 25- to
65-year age group. ABS (2018).
Australian population growth rate
1.6%
Population growth rate for the year ended 31
the
December 2017, ABS (2018)
COPD prevalence in sufferers 40-
7.5%
+/- 10%
The base estimate is from the Toelle et al. (2013)
65 years old (with symptoms;
COPD in the Australian burden of lung disease
GOLD Stage II or higher COPD)
(BOLD) study. A lower and higher estimate is
under
included in a sensitivity analysis.
(CTH) Care.
AATD deficiency genotype (types
0.63%
+/- 10%
Rahaghi et al. (2012) found 0.63% of 3,152 COPD (>
ZZ or SZ) prevalence among
GOLD II, FEV1/FVC ratio < 0.7, with post-
COPD patients
bronchodilator FEV Aged
1<80% predicted) subjects had a
1982
severe deficiency genotype.
released
Adjustment to Rahaghi et al.
90%
The Rahaghi et al. (2012) study included 0.5% Asian
Act and
(2012) to match ethnicity risk
subjects who are known to have low AATD deficiency
profile in for AATD deficiency
genotype (types ZZ or SZ) prevalence. The Australian
genotype in the Australian
been population is adjusted for the high-risk group (eg.
population
European decent) to be 90% of the population10.
has
Health
AATD 40-65 years old patients
10%
+/- 10%
In the UK around 4.6% of those with the Pi*ZZ
of
with COPD (GOLD II or higher)
genotype were estimated to be symptomatic and
that are symptomatic and
diagnos
Information ed. There is uncertainty around this parameter
diagnosed (%)
in Australia, with market research suggesting 5-10%
of
of those with the Pi*ZZ genotype would be
symptomatic and diagnosed. This is equivalent to
around10% of 40-65-year-old AATD patients with
document
COPD (GOLD II or higher) given prevalence of the pi-
ZZ genotype in Australia has been estimated at
Department approximately 1 in 5,584 by de Serres et al. (2003)
This
Proportion of 40-65 year old
Freedom
s47(1)(b)
Estimate based on proportion of non or ceased
symptomatic patients who are
smokers based on confidential market research
the
eligible
by
AT product cost
Product cost
Estimate of average, high and low prices per 1,000ml
vial
Vial size
1,000mg
(Zemaira, PROLASTIN-C Information)
Average weight kg
75.9 kg
RAPID trial
Dose
60mg/kg
(Zemaira, PROLASTIN-C Information)
10 ABS 2018. 3101.0 - Australian Demographic Statistics, December Quarter 2017, June 2018.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
136
Page 150 of 218
FOI 5155 - Document 4
Assumption
Base case
Sensitivity Reference
analysis
Adherence
s47(1)
(b)
Estimate based on confidential market research 2015
Infusions per year
52
(Zemaira, PROLASTIN-C Information)
Total annual drug cost
s47(1)(b)
Calculated, numbers of vials rounded to whole
number
Administration cost
Administration costs per infusion
$55.3
MBS item number 13915, 85% benefit for outpatient
delivery
Total annual treatment costs
s47(1)(b)
Calculated
Treatment uptake
the
Proportion of eligible patients
s47(1)(b)
Estimate based on confidential market research 2015
treated
Abbreviations:
ABS = Australian Bureau of Statistics,
AATD = alpha-1 antitrypsin deficiency,
ABS = Australian bureau of statistics,
COPD = chronic obstructive pulmonary disease,
GOLD = global initiative for chronic obstructive lung disease,
FEV1 = forced expiratory
under
volume in 1 second,
FVC = forced vital capacity,
MBS = Medical Benefit Schedule.
(CTH) Care.
There is no estimate available for the number of Australian patients with COPD due to AAT
deficiency. Rahaghi and colleagues (2012) estimated the frequency of abnormal AAT genotypes
1982 Aged
among patients with COPD11 across 19 centres in the USA. Eligible patients were offered testing for
released
AATD, with 3,457 patients being tested. Deficient patients (ZZ, S
Act Z) accounted for 0.63% of those
and
tested. Deficient patients (ZZ, SZ) constituted 0.63% of those tested, while 10.88% were carriers (MS,
been
MZ). There were lower rates of AAT deficiency among African American subjects and no incidence
amongst Asians, although only a very small number of people with Asian background (0.5%)
has
Health
participated in the study.
Information
of
Around 28% of Australia's population was born overseas. In recent years many residents have been
of
born in Asian countries, with large increases from Japan (24%), China (8%), Malaysia (7%) and India
(6%).12 Immigration estimates only provide an indication of background ethnicity as children are not
document
considered. The census includes questions about 'language spoken at home'. In Australia in 2016,
around 300 languages were identified, with about o
Department ne-fifth of Australians speaking a language other
This Freedom
than English. After English, Mandarin was the most frequently used language (2.5% of the total
the
population). In 2016, it appeared that approximately 11% of the population spoke languages from
South and East Asia (A
by BS 2016). Given that the estimated prevalence of Pi*ZZ and associated
genotypes is very low in these populations, the estimate of 0.63% of COPD sufferers having AATD
(Rahaghi et al, 2012) is adjusted by 90% to align with the AATD higher-risk group in Australia.
In the UK around 4.6% of those with the Pi*ZZ genotype were estimated to be symptomatic and
diagnosed (NIHR 2014). There is a high degree of uncertainty about the proportion of COPD (GOLD
11 >GOLD II, FEV1/FVC ratio < 0.7, with post-bronchodilator FEV1<80% predicted
12 http://www.abs.gov.au/AUSSTATS/abs@.nsf/Latestproducts/3412.0Media%20Release12015
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
137
Page 151 of 218
FOI 5155 - Document 4
Stage II or higher) patients diagnosed with the Pi*ZZ genotype in Australia. Based on confidential
market research it is estimated that 5% to 10% of those with the Pi*ZZ genotype are symptomatic
and diagnosed, which is equivalent to 10% of COPD (GOLD II or higher) 40-65 years old patients with
AATD. The proportion is subject to univariate sensitivity analysis where upper and lower estimates of
9% and 11% are included. Changing this assumption has a significant impact on the financial costs of
the listing, although product price changes have a greater impact. It is likely that clinicians would test
a larger number of patients in the event that AT were listed, therefore diagnosis rate is an important
consideration.
The budget impact approach combines the Australian population (aged 40 to 65 years), with
estimated prevalence of diagnosed COPD in patients aged over 40 years and an estimate of AATD
the
patients with diagnosed COPD (GOLD II or higher). Not al diagnosed patients would be eligible, as
many could be smokers. It is assumed that around half would be ex- or non-smokers and meet other
inclusion criteria. s45, s47(1)(b)
under Care.
(CTH)
Correspondingly s47(1)
(b)
of AT eligible patients are assumed to be prescribed AT.
s47(1)(b)
1982 Aged
E.2.1.
N
released
UMBER OF PATIENTS WITH THE MEDICAL CONDITION TARGETED BY THE PROPOSED MEDICAL
Act and
SERVICE
been
The number of patients eligible for treatment is shown in Table 83. The 25- to 65-year-old Australian
population was sourced from the ABS. The prevalence of 7.5% was derived from Toelle et al. (2013)
has
Health
who reported the prevalence of patients in Australia with COPD whose post-bronchodilator
of
FEV1/FVC ratio < 0.70 and FEV1 < 80% predicted. Estimates for 2019 predict a total of 604,009 COPD
Information
patients in this age group, increasing to 643,603 in 2023 with an Australian population growth rate
of
of 1.6%.
document
Table 83
Population eligible for augmentation therapy with A1PI in Australia
Description
2019
2020
2021
Department
2022
2023
Source
This Freedom
ABS, Australian
Australian population 25,167,031
the 25,569,704 25,978,819 26,394,480 26,816,792 demographic
Statistics
by
Australian population
ABS, Australian
growth rate
1.6%
1.6%
1.6%
1.6%
1.6%
demographic
Statistics
Australian population
ABS, Australian
40-65 years old (%)
32.0%
32.0%
32.0%
32.0%
32.0%
demographic
Statistics
Australian population
40-65 years old
8,053,450
8,182,305
8,313,222
8,446,234
8,581,373 Calculated
Australian population
Toelle et al.
with GOLD II or
7.5%
7.5%
7.5%
7.5%
7.5%
(2013) COPD in
higher COPD (%)
the Australian
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
138
Page 152 of 218
FOI 5155 - Document 4
Description
2019
2020
2021
2022
2023
Source
burden of lung
disease (BOLD)
study
Australian population
40-65 years with
COPD (GOLD II or
604,009
613,673
623,492
633,468
643,603
Calculated
higher)
AATD prevalence
among COPD (GOLD
Rahaghi et al.
I or higher) sufferers 0.57%
0.57%
0.57%
0.57%
0.57%
(2012), adjusted
(%)
by 90%.
COPD (GOLD II or
higher) sufferers with 3,425
3,480
3,535
3,592
3,649
Calculated
AATD
the The estimate is
based on 5%-
10% of those with
COPD (GOLD II or
under PiZZ in Australia
Care.
higher) patients with
(CTH)
s45, s47(1)(b)
(1/5584) being
AATD that are
diagnosed. The
diagnosed (%)
5-10% estimate is
derived from
1982 Aged confidential
released
market research.
Act
Symptomatic COPD
and
(GOLD II or higher)
patients with AATD
342
348
354
359
365
Calculated
been
diagnosis
Estimate of
has
Health
COPD/AATD
compliance with
symptomatic patients
of
s45, s47(1)(b)
inclusion criteria
who are eligible (%)
(e.g. non-smoker,
Information
for AT
emphysema
of
COPD)
AT eligible patients
Calculated
Abbreviations:
AATD = alpha-1 antitrypsin deficienc
document y,
ABS = Australian Bureau of Statistics,
AT = augmentation therapy,
COPD =
Chronic Obstructive Pulmonary Disease,
GOLD = global initiative for chronic obstructive lung disease.
Department
As noted, genotype prevalence data from Australia is limited. Rahaghi and colleagues estimated the
This Freedom
frequency of abnormal AAT genotypes among patients with COPD and found ZZ and SZ genotypes
the
accounted for 0.63% of those tested. When adjusted by 90% to account for the European at-risk
by
population in Australia, a prevalence of 0.57% is estimated. Using these proportions, a total of 3,425
COPD sufferers (Stage II or higher) are estimated to have AATD. This increases to 3,649 with
population growth by 2023.
Not all COPD sufferers (Stage II or higher) with AATD are diagnosed. In the UK (NIHR 2014), about
4.6% of those with the Pi*ZZ genotype were estimated to be symptomatic and diagnosed. Market
research in Australia suggests that somewhere between s47(1)(b) of those with AATD are diagnosed.
De Serres et al. (2003) reported gene frequencies per 1,000 persons from a range of cohort studies
conducted in various populations in Australia. PiZZ prevalence was estimated at 1 in 5,584. When
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
139
Page 153 of 218
FOI 5155 - Document 4
this prevalence is applied to the Australian population in 2019, a total of 4,507 are estimated to
carry high-risk genotypes. If s47(1)(b) is applied to this sub-population, then 342 symptomatic COPD
(GOLD II or higher) patients with AATD diagnosis are estimated for 2019.
E.2.2.
NUMBER WHO WOULD BE ELIGIBLE FOR THE REQUESTED RESTRICTION
The eligible population includes non- or previous smokers with emphysema COPD. It is estimated
that these criteria apply to s47(1)
(b)
of the symptomatic COPD (GOLD II or higher) patients with AATD
diagnosis. s47(1)(b)
A total of s47(1)
s47(1
(b)
patients are estimated to be eligible for AT in 2019, which increases to )(b)
by 2023.
the
E.2.3.
NUMBER OF PATIENTS LIKELY TO USE THE PROPOSED MEDICAL SERVICE
It is estimated that the uptake of AT will be approximately s47(1) under
(b)
by 2022, as many clinicians have
(CTH) Care.
indicated that they would prescribe AT should it become listed. The numbers of patients likely to
take up AT over a six-year period are summarised in Table 84. It is estimated that in 2019 a total of
s47(1)
s47(1)
s47(1)
1982 Aged
(b)
patients would use AT, increasing to (b) patients by 2023. This is equivalent to(b) of COPD
(GOLD II or higher) sufferers with AATD (i.e. s47(1)
released
(b)
of 1,825) in 2023.
Act and
s45, s47(1)(b)
been
has
Health
Information
of
of
E.2.4.
N
document
UMBER OF TIMES THE PROPOSED MEDICAL SERVICE IS DELIVERED OVER FIVE YEARS
AT is delivered on a per kilogram basis, at a dose of 60mg/kg/week. The estimated cost per patient is
This
Department
based on the average weight of ad
Freedom ult patients (76 kg) in the RAPID trial. This weight is similar to the
the
average adult weight of 77kg in Australia from the ABS (2012).13 Patient weight, dose per kg
recommendations, vial s
by ize and adherence assumptions are combined to estimate the number of
vials used per week across Australia. These estimates are provided in Table 85.
13 Australian Health Survey: First Results, 2011-12
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
140
Page 154 of 218
FOI 5155 - Document 4
Table 85
Estimated AT vial usage in Australia, 2019-2023
2019
2020
2021
2022
2023
Number of vials
AT patients across Australia
s45, s47(1)(b)
Average weight (kg)
76
76
76
76
76
Recommended dose of AT
60
60
60
60
60
Grams of AT per patient per week
4554
4554
4554
4554
4554
Vials per patient per week
5
5
5
5
5
Adherence
s45, s47(1)(b)
Vials per year across Australia
Abbreviations:
AT = augmentation therapy.
It is evident that s47(1) vials are estimated for 2019, increasing to s47(1) by 2023. V
the ials per patient
(b)
(b)
per week are estimated on a whole number basis as it is assumed that vial fractions cannot be held
from week to week or distributed among patients.
under
(CTH) Care.
E.1.
COSTS TO THE NBA OF THE PROPOSED THERAPY OVER FIVE YEARS
The proposed price of PROLASTIN-C is s47(1) and Zemaira s47(1) per 1,000ml vial. An average price of
1982 Aged
(b)
(b)
s47(1) is included, with s47(1) and s47(1) used as high and low bounds in sensitivity analyses. The
released
(b)
(b)
(b)
financial impact to the NBA for AT is summarised in Table 86. The
Act estimat
and ed cost is presented over
the six-year costing proposal period and is based on the s47(1)uptake rate for AT by 2023. Uptake
(b)
been
begins at s47(1) and increases by s47(1) per year. The cost to the NBA for the total AT market is
(b)
(b)
estimated to be s47(1)(b)
in 2019, increasing to s47(1)(b)
in 2023.
has
Health
of
Table 86
Estimated financial impact to the National Blood Authority; total augmentation therapy market
Information
2019
2020
2021
2022
2023
of
Number of vials
Number of vials across Australia
s45, s47(1)(b)
document
Cost per 1,000ml vial $
Cost per patient per year $
This
Department
Total cost of augmentation therapy $
Freedom
the
E.2.
CHANGES IN
by
USE AND COST OF OTHER MEDICAL SERVICES
Changes in other MBS-funded medical services likely to be affected by listing the proposed product,
are outlined in Table 87. Patients will receive MBS benefits for AT infusions. Each service is costed
using MBS item number 13915 at 85% of benefit. This unit cost is multiplied by the number of
patients and adherence to calculate aggregate MBS costs for infusion. MBS financial costs increase
from $278,000 in 2019, to $443,000 in 2023. Compared to AT product costs, these expenses are
minimal. Clinicians are also likely to screen more patients for AATD should AT be listed. Given the
rare nature of AATD, these costs are likely to be small.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
141
Page 155 of 218
FOI 5155 - Document 4
Table 87
Estimated financial impact to MBS from augmentation therapy listing
2019
2020
2021
2022
2023
Outpatient AT delivery
AT eligible patients
s45, s47(1)(b)
MBS item number 13915 per patient per year
52
52
52
52
52
MBS item number 13915 services per year
5,343
6,333
7,353
8,405
8,539
MBS benefit per service
55.3
55.3
55.3
55.3
55.3
MBS benefit per patient per year
2,876
2,876
2,876
2,876
2,876
Adherence
94%
94%
94%
94%
94%
Infusion delivery MBS costs
277,422
328,838
381,828
436,429
443,412
Abbreviations:
AT = augmentation therapy,
MBS = Medical Benefit Schedule.
E.3.
O
the
VERALL FINANCIAL IMPLICATIONS
The five-year budget impact underpinned by the assumptions above is presented in Table 88. Costs
under
increase from s47(1)(b)
in 2019 to s47(1)(b)
in 2023. Listing AT has financial implications
(CTH) Care.
for other parts of the Australian Government’s health budget, for state and territory government
health budgets including public hospitals, and for patients and private insurers. These costs were
Aged
modelled in the economic analysis in Section D. Disease management cost
1982 s for COPD are estimated
released
to be minor compared with AT product costs. More than 95% of the incremental resource cost
Act and
estimated in Section D is associated with AT. been
Table 88
Estimated financial impact to government from augmentation therapy listing
2019
2020
2021
Health
2022 2023
has
Total government costs
of
AT patients
s45, s47(1)(b)
Information
NBA-supported AT product costs
of
MBS-supported infusion service delivery
277,422
328,838
381,828
436,429
443,412
Total costs to government
s45, s47(1)(b)
document
Abbreviations:
AT = augmentation therapy,
MBS = Medical Benefit Schedule,
NBA = national blood authority.
Department
E.4.
IDENTIF
This
ICATION, ESTIMATION AND REDUCTION OF UNCERTAINTY
Freedom
the
The budget impact model presented in this section provided a base case in which the lower estimate
by
was utilised. Key base assumptions are included in a sensitivity analysis in Table 89. The budget
impact is most sensitive to the assumed price for AT and the age grouping of the Australian
population. The overal budget varies by 15% under the high- and low-price assumptions. Given the
large contribution of the AT product itself to overall resource in the economic model, variations in
price have a large impact on both financial and economic attractiveness.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
142
Page 156 of 218
FOI 5155 - Document 4
Table 89
Net government cost sensitivity analysis
Year 1
Year 2
Year 3
Year 4
Year 5
Base case net cost
s45, s47(1)(b)
s47(1)(b)
Australian population 25-65 years old,
13,215,161 people
COPD prevalence in sufferers 40-65 years
old (with symptoms; GOLD Stage II or higher
COPD), 6.8%
COPD prevalence in sufferers 40-65 years
old (with symptoms; GOLD Stage II or higher
COPD), 8.3%
the
AATD prevalence among COPD (GOLD II or
higher) patients 0.51%
AATD prevalence among COPD (GOLD II or
higher) patients 0.62%
under Care.
AATD patients with COPD (GOLD II or
(CTH)
higher) that are symptomatic and diagnosed
(9%)
AATD patients with COPD (GOLD II or
1982 Aged
higher) that are symptomatic and diagnosed
released
(11%)
Act and
COPD/AATD symptomatic patients who are
eligible (%) 45%
been
COPD/AATD symptomatic patients who are
eligible (%) 55%
has
Health
s47(1)(b)
Information
of
Abbreviations:
AATD = alpha-1 antitrypsin deficiency;
AT = augmentation therapy;
COPD = Chronic Obstructive Pulmonary Disease;
GOLD = global initiative for chronic obstructive lung disease;
MBS = Medical Benefit Schedule.
of
The base budget impact was estimated using the 40- to 65-year-old population in Australia. AATD is
document
likely to present within this age bracket, as the average ages at baseline in most trials have been in
the early 50s. Given AATD life expectancy, there are likely to be limited patient numbers above age
This
Department
65. Some patients may present earlie
Freedom r than 40, therefore COPD/AATD prevalence is also estimated
the
for the 25- to 65-year-old age group. The budget impact is large when this age group is included. This
scenario over-estimates th
by e eligible population, given that most patients suffer severe COPD in their
50s. The scenario indicates that usage by younger age groups than that in the base scenario would
have significant budget impacts.
Financial impact is also sensitive to varying the prevalence proportions by 10%, however, less so
than the upper and lower price estimation. Uptake rate also has an impact. A decrease in year 2022
uptake froms47(1)(b)
results in a s47(1)(b) budget requirement in that year. Further sensitivity
analyses to parameters used in deriving budget impact estimates presented in this Section can be
performed with the attached spreadsheet (‘Alpha_Budget Impact_FINAL.xlsx’).
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
143
Page 157 of 218
FOI 5155 - Document 4
SECTION F
OTHER RELEVANT CONSIDERATIONS
ACCESS CONSIDERATIONS
The use of blood products is routinely governed/administered through specialised centres, which
can limit the availability and access of A1PI to patients living within major metropolitan areas.
Further, treatment must be initiated and monitored by a respiratory physician (PROLASTIN®-C
product summary), which can impact the ability of rural and remote patients to obtain and use the
drug. In order to be tested for AATD and potential y receive A1PI, patients must initially travel to
these centres, incurring additional costs such as childcare, accommodation, travel, and time away
the
from work. Clinical feedback suggests that once patients have been deemed eligible for A1PI, they
can access the drug via a local pharmacy or outpatient clinic.
under
The proposed shelf life of lyophilised A1PI is 36 months at ≤ 25Co, with the reconstituted p
Care.roduct
(CTH)
requiring administration within three hours (Australian Public Assessment Report for A1PI).
Consequently, A1PI needs to be transported using a temperature-control ed supply chain (i.e. cold
1982 Aged
chain), which will incur a cost. It is unclear whether temperature controlled transport is accessible to
released
rural patients. Clinical feedback suggests that no cold supply chains are currently established due to
Act and
the limited funding of A1PI, but that these challenges are not insurmountable.
been
Patients can administer A1PI at home or with the assistance of a carer, when deemed appropriate by
Health
the treating specialist and after receiving ad
has equate training (PICO Confirmation, page 11). Specific
training required to become competent in A1PI self-adm
of inistration needs to be specified, as this will
Information
incur additional costs and resources.
of
It is unclear whether eligibility to receive A1PI should be granted to AATD patients who have
previously received lung transplants
document . Given that AATD is a genetic disorder, the replaced lungs will
be gradually damaged, necessitating another transplant. By granting access to this group of patients,
Department
it is anticipated tha
This t the transplant’s longevity will be increased.
Freedom
the
Given the progressive nature of AATD, it is anticipated that early intervention with A1PI would result
by
in greater long-term lung health. However, clinical trial populations have only included severe
patients. It is unclear whether less severe patients should have access to this drug as well.
The PICO Confirmation noted that clinical experts advise that cigarette smoking inactivates A1PI,
rendering this expensive product useless in smokers. Excluding smoking patients may restrict access
of A1PI to specific communities. To mitigate any potential discrimination of usage, it is suggested
that smoking patients in specific communities be provided with the resources required to quit, in
order to become eligible.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
144
Page 158 of 218
FOI 5155 - Document 4
DOSING CONSIDERATIONS
The PROLASTIN-C product summary recommends administering 60mg/kg of the drug intravenously
once a week (PROLASTIN-C product summary). However, the precise dose that confers the greatest
clinical efficacy is yet to be determined. Several published trials have examined alternate doses of AT
with the primary outcome either the number of adverse events or peak/trough concentration of
A1PI in serum (Table 90). The only published study comparing different doses of AT concluded that
steady-state serum concentration of A1PI was higher following 120mg/kg compared to 60mg/kg and
the number of adverse events was similar between the two treatments (Campos et al. 2013). No
study has evaluated the clinical efficacy between different doses of AT. A clinical trial (SPARTA)
comparing 120mg/kg A1PI, 60mg/kg A1PI and placebo on lung density is expected to finish in 2021.
the
Table 90
Studies evaluating different doses of A1PI therapies
Study
Location, follow-up,
Dose(s)
Adverse events
under
patient numbers
(CTH) Care.
Single-arm studies
Pi tulainen et al. (2003)
Sweden
120mg/kg every 2 weeks
Not reported
Case series
4 weeks
1982 Aged
Level IV
N = 5
released
Hubbard and Crystal (1988) United States
250mg/kg every 28 days
No adverse events
Act and
Case series
12 months
Level IV
N = 9
been
Comparative studies
Sorrells et al. (2015)
15 countries
120mg/kg vs 60mg/kg vs
Not applicable
has
Health
(SPARTA trials)
160 weeks
placebo weekly
Ongoing trial
of
RCT
N = 339 (estimated)
Level II
Information
Campos et al. (2013)
United States
of
60mg/kg weekly vs
69 and 43 TAE in 60 and
RCT
22 weeks
120mg/kg weekly
120mg/kg respectively
Cross over
N = 30
document
Level II
Dirksen et al. (1999)
Denmark and The
250mg/kg vs albumin
No adverse events
Department
RCT
Netherlands
(625mg/kg) every 4 weeks
This Freedom
Level II
At least 36 months
the
N = 56
Abbreviations:
mg/kg = mil igrams per kilogram of body weight,
RCT = randomised controlled trials, TAE = treatment emergent adverse
by
events.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
145
Page 159 of 218
FOI 5155 - Document 4
ETHICAL CONSIDERATIONS: RULE OF RESCUE
The following four criteria outline eligibility for a service to be considered under the rule of rescue:
1. NO ALTERNATIVE TREATMENT OPTION EXISTS IN AUSTRALIA
There is currently no effective disease-modifying treatment for patients with AATD. Current
treatments address the symptoms but not the cause of AATD. AT with A1PI is the only potential
treatment for patients with AATD. Therefore, A1PI fulfils this criterion.
2. THE MEDICAL CONDITION IS SEVERE, PROGRESSIVE AND EXPECTED TO LEAD TO PREMATURE DEATH
The pathogenesis of AATD results in the progressive deterioration of an individual’s lungs. This
the
causes significant morbidity and premature death among those afflicted with the disorder. A1PI has
the potential to slow disease progression in patients with AATD. Therefore, A1PI fulfils this criterion.
under
3.
Care.
THE MEDICAL CONDITION APPLIES TO A VERY SMALL NUMBER OF PATIENTS
(CTH)
The medical condition defined by the requested restriction applies to only a very smal number of
patients. Again, the fewer the patients, the more influential the rule of rescu
1982 e might b
Aged e in the PBAC’s
consideration. However, the PBAC is also mindful that the PBS is a c
released ommunity-based scheme and
Act
cannot cater for individual circumstances.
and
been
Based on estimates by commercial sponsors, the incidence of people meeting the criteria for A1PI in
Australia in 2018 was s47(1
Health
)(b)
(PICO Confirmation page 4). s47(1)(b)
has
Information
of
4. THE PROPOSED SERVICE PROVIDES A WORTHWHILE CLINICAL IMPROVEMENT
of
It is unclear whether A1PI fulfils this criterion. The primary outcome in the included RCTs was change
in lung density, inferred by CT den
document sitometry. CT lung density has been suggested to be a more
sensitive measure of mortality than FEV1, thereby requiring smaller sample sizes in order to detect
Department
meaningful differen
This ces in a clinical trial setting (Schluchter et al. 2000).
Freedom
the
Overall, the literature base suggests that lung CT densitometry is correlated to functional outcomes
by
including FEV1, KCO and mortality. It is less clear whether it is correlated to QoL. However, when
these correlations are taken together with the findings from the trials listed in section B, it is unclear
whether A1PI fulfils this criterion.
In order to detect statistically significant differences between placebo and AT, both Stockely et al.
(2018) and Schluchter et al. (2000) have recommended large sample sizes. Owing to the rarity of
AATD it is unclear whether obtaining such numbers would be feasible.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
146
Page 160 of 218
FOI 5155 - Document 4
APPENDIX A
CLINICAL EXPERTS AND ASSESSMENT
GROUP
CLINICAL EXPERTS CONSULTED DURING THE PREPARATION OF THIS REPORT
Name
Expertise
s47F
the
under Care.
A
(CTH)
SSESSMENT GROUP
Research and Evaluation incorporating ASERNIP-S, Royal Australasian Col ege of Surgeons, South
1982 Aged
Australia
released
Act
Name
Position
and
s47F
been
has
Health
Information
of
of
document
This Freedom
Department
the
Noted conflicts of in
by
terest
The assessment group has no conflicts of interest to report.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
147
Page 161 of 218
FOI 5155 - Document 4
APPENDIX B
SEARCH STRATEGIES
Bibliographic databases
Electronic database
Time period searched
Results
Embase
Inception – 23 May 2018
4412
PubMed
Inception – 24 May 2018
3221
The Cochrane Library (CDSR, Central, DARE, HTA, HEED)
Inception – 24 May 2018
67
Additional sources of literature (including websites)
the
Source
Location
Search date
Clinical trial registries
under
ClinicalTrials.gov
https://clinicaltrials.gov/
28 May
Care. 2018
(CTH)
Cochrane Central Register of Controlled Trials
http:/ cochranelibrary-
28 May 2018
wiley.com/cochranelibrary/search
EU Clinical Trials Registry
https://www.clinicaltrialsregister.e
1982 u/ctr-
Aged 29 May 2018
search/search
released
WHO International Clinical Trials Registry Platform
http://www.who.int/ictrp/en/
29 May 2018
Act and
(ICTRP)
Current Controlled Trials MetaRegister
http://www.isrctn.com
29 May 2018
been
Australian New Zealand Clinical Trials Registry
http://www.anzctr.org.au/
29 May 2018
Grey literature sources
Health
has
New York Academy of Medicine Grey Literature Report http://www.greylit.org
24 May 2018
of
Economic studies
Information
CEA Registry
https://research.tufts-nemc.org/cear4/
13 June 2018
of
HTA Websites
National Information Centre of Health Services
http://www.nlm.nih.gov/nichsr/
24 May 2018
document
Research and Health Care Technology (NICHSR)
National Library of Medicine Health
http://www.ncbi.nlm.nih.gov
24 May 2018
Department
Services/Technology Assessment Texts (HSTAT)
This Freedom
International Information Network on New and
http:/ euroscan.org.uk/
24 May 2018
the
Emerging Health Technologies (EuroScan International
Network)
by
Other sources
National Institute for Heath and Care Excellence
http://www.nice.org.uk
24 May 2018
(NICE)
NHS National Institute for Health Research (NIHR),
http://www.nets.nihr.ac.uk/programmes/hta
24 May 2018
including HTA programme
Online Mendelian Inheritance in Man
http://omim.org/
24 May 2018
Patient/practitioner societies
Alpha-1 Foundation
http://www.alpha1.org/Investigators/Resourc
24 May 2018
es/Last-Month-on-PubMed
National Blood Authority
http:/ www.blood.gov.au
24 May 2018
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
148
Page 162 of 218
FOI 5155 - Document 4
Source
Location
Search date
Lung Foundation Australia
http://www.lungfoundation.com.au
24 May 2018
Thoracic Society of Australia and New Zealand
http://www.thoracic.org.au/
24 May 2018
Manufacturers
Grifols
http://www.grifols.com/en/web/international/h
24 May 2018
ome#
CSL Behring
http:/ www.cslbehring.com.au/
24 May 2018
SHIRE
http://www.shire.com
24 May 2018
Extended assessment of harms
Medsafe Recall Actions Archive
http://www.medsafe.govt.nz/
16 July 2018
Medsafe Early Warning System Alert Communications http://www.medsafe.govt.nz/
16 July 2018
MBS Online
http:/ www.mbsonline.gov.au/
the 16 July 2018
Therapeutic goods administration (TGA) Current Year
https:/ www.tga.gov.au/current-year-alerts
16 July 2018
Alerts
Therapeutic goods administration (TGA) Recalls
https://www.tga.gov.au/recal s
16 July 2018
under Care.
The Pharma Letter
https:/ www.thepharmaletter.com/listing/phar
(CTH) 16 July 2018
maceutical/respiratory-and-pulmonary-
FDA Recalls
https:/ www.fda.gov/BiologicsBloodVaccines/
16 July 2018
SafetyAvailability/Recalls/default.htm
1982 Aged
FDA Device Advice
https:/ www.fda.gov/medicaldevices/devicere
16 July 2018
gulationandguidance/defaul
released t.htm
European Commission Market Surveil ance and
https:/ ec.europa.eu/
Act
16 July 2018
and
Vigilance
been
Table 91
PubMed Search Strategy has
Health
Search
Searched terms
Results
of
#1
alpha 1-Antitrypsin Deficiency[MeSH Terms]
3221
Information
#2
alpha 1-Antitrypsin Deficiency of
4,480
#3
alpha1-proteinase inhibitor deficiency
4,493
document
#4
#1 or #2 or #3
3,221
Department
#5
Chronic obs
This tructive pulmonary disorder
50,532
Freedom
#6
Chronic obstructive pulmonar
the y disorder[MeSH]
48,147
#7
COPD
by
75,911
#8
COPD[MeSH]
48,245
#9
Emphysema
34,561
#10
Emphysema[MeSH]
26,463
#11
#5 or #6 or #7 or #8 or #9 or #10
96,691
#12
#4 and #11
3,221
Databases searched: PubMed
Restrictions: Humans; English
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
149
Page 163 of 218
FOI 5155 - Document 4
Search
Searched terms
Results
Date searched: 24 May 2018
Total results: 3221
Table 92
Embase Search Strategy
Search Searched terms
Results
#1
alpha 1-Antitrypsin Deficiency
11,139
#2
Alpha 1 antritrypsin deficiency
11,148
#3
alpha1-proteinase inhibitor deficiency
32
#4
#1 or #2 or #3
11,167
the
#5
Chronic obstructive pulmonary disorder
1,134
#6
COPD
160,580
under
#7
Emphysema
105,210
(CTH) Care.
#8
#5 or #6 or #7
249,005
#9
#4 and #8
4,412
1982 Aged
Databases searched: Ovid Embase; Ovid Medline released
Restrictions: Humans; English
Act and
Date searched: 23 May 2018
Total results: 4412
been
Health
Table 93
Cochrane Search Strategy has
of
Search Searched terms
Results
Information
alpha 1-Antitrypsin Deficiency
106
of
#2
alpha1-proteinase inhibitor deficiency
24
document
#3
#1 or #2
115
#4
Emphysema
1107
This
Department
#5
COPD
Freedom
11127
the
#6
#4 or #5
12065
#7
#3 and #6 by
67
Databases searched: Cochrane Database of Systematic Reviews
Date searched: 24 May 2018
Total results: 67
Table 94
Clinicaltrials.gov Search Strategy
Search Searched terms
Results
#1
Alpha 1-Antitrypsin Deficiency
76
#2
Zemaira
156
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
150
Page 164 of 218
FOI 5155 - Document 4
Search Searched terms
Results
#3
Prolastin
156
#4
#1 and (#2 or #3)
76
Date searched: 28 May 2018
Total results: 76
Table 95
Cochrane Central Register of Control ed Trials Search Strategy
Search Searched terms
Results
#1
Alpha 1-Antitrypsin Deficiency
118 the
Date searched: 28 May 2018
Total results: 118
under
(CTH) Care.
Table 96
EU Clinical Trials Registry Search Strategy
Search Searched terms
Results
1982 Aged
#1
Alpha 1-Antitrypsin Deficiency
10
released
Date searched: 29 May 2018
Act
and
Total results: 10
been Health
Table 97
WHO International Clinical Trials Registry Platform Search Strategy
has
of
Search Searched terms
Results
Information
#1
Alpha 1-Antitrypsin Deficiency
67
of
Date searched: 29 May 2018
Total results: 67
document
This Freedom
Department
Table 98
Current Control ed Trials MetaRegister Search Strategy
the
Search Searched terms
Results
by
#1
Alpha 1-Antitrypsin Deficiency
8
#2
Zemaira
0
#3
Prolastin
0
Date searched: 29 May 2018
Total results: 8
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
151
Page 165 of 218
FOI 5155 - Document 4
Table 99
Australian New Zealand Clinical Trials Registry Search Strategy
Search Searched terms
Results
#1
Alpha 1-Antitrypsin Deficiency
2
Date searched: 29 May 2018
Total results: 2
Table 100
CEA Registry Search Strategy
Search Searched terms
Results
#1
Alpha 1-Antitrypsin Deficiency
8 the
Date searched: 13 June 2018
Total results: 2
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
152
Page 166 of 218
FOI 5155 - Document 4
APPENDIX C
STUDIES INCLUDED IN THE SYSTEMATIC REVIEW
Table 101
Characteristics of randomised control ed trials included in the systematic review to assess ef icacy
the
Authors
Study design Location
Study population
Description of
Description of
Relevant outcomes assessed
Measurement of
Publication Year Evidence
Length of
characteristics (at baseline Intervention
Comparator
outcomes and
Study ID
level
follow-up
after randomisation)
analysis
Chapman et al. RCT
21-centred trial
n
under
α1-antitrypsin = 93
Intervention
Comparator
Primary outcome
Primary outcome
Care.
2015
(CTH)
Level: IIA
across 13
nplacebo = 87
Intravenous A1PI
Placebo
Lung density reduction rate measured by Analysed by GLMM
RAPID
countries
Age
60mg/kg per week No further detail
PD15 for TLC and FRC combined and
where TLC, FRC,
[I] = 53.8 ± 6.9
for 24 months (12
provided
separately at baseline, 3 months and 12 country, time,
24 months
Aged
[C] = 52.4 ± 7.8
months masked and
months
treatment and
1982
masked period
12 months opened)
treatment-by-time
released
and up to 48
FEV1 Predicted (%)
Secondary outcome
interaction using
Act
month
[I] = 47.4 ± 12.1
fixed effects and
and •
extension with [C] = 47.2 ± 11.1
Anthonisen exacerbation
patients and time-by-
open-label
Alpha-1 Antitrypsin (μM)
• Exacerbation duration and severity
patient using random
been
[I] = 6.38 ± 4.62
• FEV1
effects
[C] = 5.94 ± 2.42
• Single-breath dif usion capacity
has
Health
Lung density (g/L)
• A1PI concentration
of
TLC-[I] = 45.5 ± 15.8
• Incremental shuttle walk test
Information
TLC-[C] = 48.9 ± 15.5
• SGRQ status
FRC-[I] = 47.6 ± 15.7
• BMI
of
FRC-[C] =50.7 ± 15.0
• Mortality
Comb-[I] = 46.6 ± 15.6
• TEAE
document
Comb-[C] = 49.8 ± 15.1
Genotype (both arms)
Department
PiZZ = 168 (93%)
This Freedom
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
153
Page 167 of 218
FOI 5155 - Document 4
Authors
Study design Location
Study population
Description of
Description of
Relevant outcomes assessed
Measurement of
Publication Year Evidence
Length of
characteristics (at baseline Intervention
Comparator
outcomes and
Study ID
level
follow-up
after randomisation)
analysis
Dirksen et al.
RCT
Denmark, UK
nα1-antitrypsin = 38
Intervention
Placebo
Primary outcome
Four methods were
2009
Level: IIA
and Sweden
nplacebo = 39
Intravenous A1PI
2% albumin, same Lung density reduction rate measured by used:
the
EXACTLE
Age
60mg/kg per week dosage to the
PD15 via four dif erent methods at 12, 24 • Method 1: GLMM
Up to 30
[I] = 54.7 ± 8.4
for 24 months
intervention
and 30 months;
without lung volume
months
[C] = 55.3 ± 9.8
variable, this was
regarded as the
Gender (M:F)
Secondary outcome
under
primary outcome
[I] 25:13
• FEV
Care.
1
(CTH)
among the other
[C] 16:23
• Dif usion capacities (DLco)
three;
FEV1 Predicted (%)
• Transfer coefficient (KCO)
• GLMM with lung
[I] = 46.3 ± 19.6
• Exacerbation duration and severity
volume variable;
1982 Aged
[C] = 46.6 ± 21.0
defined by Rodriguesz-Roisin criteria • ANCOVA without
released
Alpha-1 Antitrypsin
• SGRQ status
lung volume as a
Act and
concentration (μM)
• AE
covariate;
[I] = 4.6 ± 1.6
• ANCOVA with lung
been
[C] = 4.6 ± 1.7
volume as a
Lung density (g/L)
covariate
Health
TLC-[I] = 54.55 ± 13.37 has
TLC-[C] = 53.90 ± 15.97
of
Comb-[I] = 47.98 ± 19.07
Information
Comb-[C] = 45.48 ± 46.95 of
Genotype (both arms)
PiZZ = 77 (100%)
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
154
Page 168 of 218
FOI 5155 - Document 4
Authors
Study design Location
Study population
Description of
Description of
Relevant outcomes assessed
Measurement of
Publication Year Evidence
Length of
characteristics (at baseline Intervention
Comparator
outcomes and
Study ID
level
follow-up
after randomisation)
analysis
Dirksen et al.
RCT
Denmark and
nα1-antitrypsin = 28
Intravenous A1PI
625mg/kg albumin,
Lung density parameters including
GLMM on all
1999
Level IIA
The
nplacebo = 28
250mg per month for same frequency to • Whole lung density by PD15
outcomes where
the
DIRKSEN99
Netherlands
Age*
at least 36 months the intervention
• Lung density slice 5cm below the
fixed effects were
Overall mean = 47.56
carina
time, nations, lung
At least 36
volume (log-
Gender (M:F)
months
transformed) and
Overall = 34:22
under
treatment arms, and
FEV
(CTH) Care.
1 Predicted (%)
Lung function parameters including random effects were
[I] = 46.2 ± 11.90
• FEV1 and %predicted
patients IDs
[C] = 50.0 ± 15.93
• FVC
Lung density (whole lung,
•
1982 Difusion c
Aged apacities (DLco)
g/L)
• Transfer coefficient (KCO)
released
[I] = 67.7 ± 22.06
Act and
[C] = 73.0 ± 28.29
Genotype (both arms)
been
PiZZ = 56 (100%)
Abbreviations:
AE = adverse events,
ANCOVA = Analysis of covariance,
BMI = body mass index,
C = comparator group,
Comb = combined,
DLco = dif using capacity for carbon monoxide,
FEV1 = forced
expiratory volume in one second,
FRC = functional residual capacity,
FVC = forced vital capacity,
GLMM = generalised linear mixed model
Health ,
I = intervention group,
KCO = DLCO divided by alveolar volume,
PD15 =
has
15th percentile point,
SGRQ = St George’s Respiratory Questionnaire,
TEAE = Treatment-emergent adverse events,
TLC = total lung capacity.
of
* The study reported some baseline parameters by centres, not by arms. Only mean values were calculable from available data and those are only for overall average, not by arms.
Information
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
155
Page 169 of 218
FOI 5155 - Document 4
Table 102
Characteristics of RCT studies used in the systematic literature review to assess safety
Authors
Study design Location
Study population
Description of Intervention
Description of Comparator
• Safety outcomes assessed
Publication Year Evidence
Length of follow-up
characteristics (at baseline
Study ID
level
after randomisation)
Chapman et al. RCT
21-centred trial across 13
Zemaira = 93
Intervention
Comparator
• Any adverse events
the
2015
Level: IIA
countries
Placebo = 87
Intravenous A1PI 60mg/kg
Placebo
• Severe adverse events
RAPID
Age
per week for 24 months (12 No further detail provided
• Treatment-related adverse
24 months masked period [I] = 53.8 ± 6.9
months masked and 12
events
and up to 48 month
[C] = 52.4 ± 7.8
months opened)
under
•
extension with open-label
Dyspnoea
(CTH) Care.
FEV
1 Predicted (%)
• Death due to adverse event
[I] = 47.4 ± 12.1
• Discontinuation due to
[C] = 47.2 ± 11.1
adverse events
Alpha-1 Antitrypsin (μM)
1982 Aged
[I] = 6.38 ± 4.62
released
[C] = 5.94 ± 2.42
Act and
Lung density (g/L)
TLC-[I] = 45.5 ± 15.8 been
TLC-[C] = 48.9 ± 15.5
FRC-[I] = 47.6 ± 15.7
has
Health
FRC-[C] =50.7 ± 15.0
of
Comb-[I] = 46.6 ± 15.6
Information
Comb-[C] = 49.8 ± 15.1
Genotype (both arms)
of
PiZZ = 168 (93%)
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
156
Page 170 of 218
FOI 5155 - Document 4
Authors
Study design Location
Study population
Description of Intervention
Description of Comparator
• Safety outcomes assessed
Publication Year Evidence
Length of follow-up
characteristics (at baseline
Study ID
level
after randomisation)
Dirksen et al.
RCT
Denmark, UK and Sweden
Prolastin = 38
Intervention
Placebo
• Adverse events
2009
Level: IIA
Placebo = 39
Intravenous A1PI 60mg/kg
2% albumin, same dosage to
experienced by >1 patient
the
EXACTLE
Up to 30 months
Age
per week for 24 months
the intervention
• Severe adverse events
[I] = 54.7 ± 8.4
• Treatment-related adverse
[C] = 55.3 ± 9.8
events
Gender (M:F)
• Dyspnoea
under
[I] 25:13
Care. •
(CTH)
Discontinuation due to
[C] 16:23
adverse event
FEV1 Predicted (%)
[I] = 46.3 ± 19.6
1982 Aged
[C] = 46.6 ± 21.0
released
Alpha-1 Antitrypsin
Act and
concentration (μM)
[I] = 4.6 ± 1.6
been
[C] = 4.6 ± 1.7
Lung density (g/L)
Health
TLC-[I] = 54.55 ± 13.37
has
TLC-[C] = 53.90 ± 15.97
of
Comb-[I] = 47.98 ± 19.07
Information
Comb-[C] = 45.48 ± 46.95
of
Genotype (both arms)
PiZZ = 77 (100%)
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
157
Page 171 of 218
FOI 5155 - Document 4
Authors
Study design Location
Study population
Description of Intervention
Description of Comparator
• Safety outcomes assessed
Publication Year Evidence
Length of follow-up
characteristics (at baseline
Study ID
level
after randomisation)
Dirksen et al.
RCT
Denmark and The
Prolastin = 28
Intravenous A1PI 250mg/kg 625mg/kg albumin, same
• Any adverse events
1999
Level: IIA
Netherlands
Placebo = 28
per month for at least 36
frequency to the intervention
the
DIRKSEN99
Age
months
At least 36 months
Overall mean = 47.56
Gender (M:F)
Overall = 34:22
under
FEV
(CTH) Care.
1 Predicted (%)
[I] = 46.2 ± 11.90
[C] = 50.0 ± 15.93
Lung density (whole lung,
1982 Aged
g/L)
released
[I] = 67.7 ± 22.06
Act and
[C] = 73.0 ± 28.29
Genotype (both arms) been
PiZZ = 56 (100%)
Abbreviations:
AE = adverse events,
C = comparator group,
I = intervention group,
RCT = randomised controlled trial,
TEAE = Treatment-emergent adverse events.
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
158
Page 172 of 218
FOI 5155 - Document 4
Table 103 Characteristics of non-randomised control ed trials used in the systematic literature review to assess efficacy
Authors
Study design Location
Study population
Description of
Description of
Relevant outcomes
Measurement of
Publication
Evidence
Length of follow-up mean characteristics (at baseline) Intervention
Comparator
assessed
outcomes and analysis
Year
level
(SD)
Study ID
the
Alpha-1-
Non-RCT
United States
Age
Intervention
Comparator
Primary outcomes
FEV1: linear mixed
Antitrypsin
Level II -3
Median (range), 52 (12 –
46 ± 11
Augmentation therapy Untreated
• ΔFEV1
effects modelling
Deficiency
86) months
Gender (M:F)
No further details
No further details
(covariates: mean
Registry Study
• Survival
provided
provided
FEV1 % predicted)
under
Group 1998
510:417
Care.
Tobacco exposure (both)
Survival: kaplan-meier,
(CTH)
log-rank test, cox
Nonsmokers = 198/927
Always receiving AT Never receiving AT
proportional hazards
Exsmokers = 655/927
N=389
N=277
regression (covariates:
Current smokers = 75/927
Partly receiving AT 1982 Aged
baseline FEV1 % and
FEV1 Predicted (%)
N=261
time)
released
49 ± 30
Act and
Alpha-1 Antitrypsin (μM)
5.7
± 1.4
been
Barros-Tizón et Non-RCT
Spain
Age
Intervention
Comparator
Primary outcome
T-test, Wilcoxon signed-
al. 2012
Level IV (pre- Total 36 months
51.7 ± 9.1
Augmentation therapy No treatment (pre-
• Number of
rank, ANOVA,
has
Health
and post-
18 months pre- and post-
FEV1 predicted
Mean dose 60.3 (3.8) intervention)
exacerbations
multivariate logistics
intervention)
of
intervention
46.0 ± 13.4
mg/kg
No further details
Secondary outcomes regression
Information
AAT serum concentrations Administered
provided
• Lung function
<11 µml (inclusion c
of riteria) Biweekly, n=22
• Adverse events
Tobacco exposure
Weekly, n=8
• Costs associated
Smokers 4/127 (3%)
Every 3 weeks, n=97
with hospitalisation
document
Exsmokers 100/127 (94%)
Nonsmokers 22/127 (17%)
Department
Genotype
This Freedom
PiZZ 118/127 (96%)
the
PiSZ 1/127 (0.8%)
Other 7/127 (6%)
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
159
Page 173 of 218
FOI 5155 - Document 4
Karl et al. 2017 Non-RCT
Germany
Number of patients
Intervention
Comparator
Primary outcomes
T-test, chi squared test,
Level II -2
1 year
[I] = 106
Augmentation therapy Untreated
• Direct and indirect Wilcoxon signed-rank
[C] = 25
No further details
No further details
health care costs tests, generalised linear
Age, mean (SD)
provided
provided
• Health-related
model (covariates:
GOLD grade, age, sex,
[I] = 59.6 (9.9)
N=106
N=25
quality of life
education, smoking
the
[C] = 63.1 (10.2)
status, BMI,
Gender (M:F)
comorbidities)
[I] = 66:40
[C] = 8:17,
p < 0.01
under
COPD GOLD grade
(CTH) Care.
[I] Grade 1 = 4.7%; Grade 2 =
24.5%
Grade 3 = 50.9%; Grade 4 =
1982 Aged
19.8%
[C] Grade 1 = 4%; Grade 2 =
released
76%
Act and
Grade 3 = 8%; Grade 4 = 12%
Education
been
[I] Basic = 33%
Secondary = 42.5%
has
Health
Higher = 24.5%
of
[C]
Basic = 28% Information
Secondary = 48%
Higher = 24%
of
Tobacco exposure [I] Never = 20.8%
document
Former = 78.3%
Current = 0.9%
Department
[C] Never = 36%
This Freedom
Former = 64%
the Current = 0%
BMI (kg/m2)
by [I] = 24.6 (4.1)
[C] = 24.8 (3.6)
Comorbidities, count mean
Alpha-1 proteinase inhibitor augmentation – MSAC
(SDC
) A 1530
160
[I] = 3.0 (2.5)
[C] = 3.4 (2.3)
Page 174 of 218
FOI 5155 - Document 4
Lieberman 2000 Non-RCT
United States
Number of patients
Intervention
Comparator
Primary outcome
Chi squared test
Level II -2
N/A
[I] = 96
Augmentation therapy Untreated
• Number of
[C] = 47
No further details
No further details
infections
Age, median (range)
provided
provided
Secondary outcomes
[I] Male = 50 (36 – 67)
N=96
N=47
• Perceived benefit
the
Female = 53 (33 – 72)
[C] Male = 55 (37 – 70)
Female = 45 (33 – 67)
Gender (M:F)
under
[I] = 50:46
(CTH) Care.
[C] = 24:23
Phenotype
[I] PiZZ = 95/96; PiSZ = 1/96
1982 Aged
[C] PiZZ = 46/47; PiSZ = 1/47
released
Tobacco exposure
Act and
[I] Exsmokers = 93/96
Never smoked = 3/96 been
[C] Exsmokers = 35/47
Never smoked = 12/47**
Health
Alpha-1 Antitrypsin
has
(60mg/kg)
of
Frequency
Information
Bi weekly = 35 of
Weekly = 54
Monthly = 7
Duration
document
< 1 year = 7
> 1 to < 10 years = 89
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
161
Page 175 of 218
FOI 5155 - Document 4
Seersholm et al. Non-RCT
Denmark and Germany
Number
Intervention
Comparator
Primary outcome
Random effects
1997
Level II -3
[I] = 198
60mg/kg per week
Untreated
• ΔFEV
modelling (covariates:
1 (mL/year)
3.2 (1.6) years for AT
[C] = 97
N=198
No further details
age at baseline, follow-
patients
Gender (M:F)
provided
up time, treatment,
gender, initial FEV
[I] = 142:56
N=97
1,
individual patients)
5.8 (3.4) years for no AT
the
[C] = 55:42,
p = 0.01
patients
Age, mean (SD)
[I] = 46 (8)
[C] = 45 (10)
under
Phenotype
(CTH) Care.
[I] PiZZ = 198/198
[C] PiZZ = 97/97
FEV1 predicted %
1982 Aged
[I] = 37 (14)
released
[C] = 42 (10),
p = 0 .02
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
162
Page 176 of 218
FOI 5155 - Document 4
Tonelli et al.
Non-RCT
United States
Number of patients
Intervention
Comparator
Primary outcome
T-test, chi squared test,
2009
Level II -3
[I] = 124
Augmentation therapy Untreated
• ΔFEV
Fisher exact test
1 (mL/year)
41.7 (2.6) months
[C] = 40
No further details
No further details
Random effects
Age, mean (SE)
provided
provided
Secondary outcomes modelling (covariates:
[I] = 61.3 (0.7)
N=124
N=40
gender, age at baseline,
• Mortality
smoking status,
the
[C] = 56.1 (1.9),
p = 0.01
individual patient, follow-
Gender M:F
up duration)
[I] = 65:59
Logistic regression
[C] = 20:20
(covariates: age, gender,
under
Ethnicity (white)
baseline FEV1, COPD,
(CTH) Care.
[I] = 95.2%
smoking status)
[C] = 90%
Tobacco exposure
1982 Aged
[I] Exsmokers = 84.7%
released
Current = 0%
Act and
[C] Exsmokers = 62.5%,
p <
0.001
Current = 5%
been
Comorbidities
[I] COPD = 37.1%
has
Health
[C] COPD = 15%,
p = 0.009
of
Baseline FEV1 (L/m), mean
Information
(SE)
[I] = 1.4 (0.1) of
[C] = 2.4 (0.2),
p < 0.001
FEV1 (% of predicted), mean
document
(SE)
[I] = 43 (2)
Department
[C] = 77 (5),
p < 0.001
This Freedom
Phenotype
the
[I] PiZZ = 124/124
[C] PiZZ = 40/40
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
163
Page 177 of 218
FOI 5155 - Document 4
Wencker et al. Non-RCT
Germany
Gender (M:F)
Intervention
Comparator
Primary outcome
T-test, chi squared test,
1998
Level IV (pre-
62:34
60mg/kg per week
No treatment (pre-
• ΔFEV
Wilcoxon signed-rank,
1 (mL/year)
and post-
98.9 (36.6) months
Phenotype
N=96
intervention)
mixed effects modelling
intervention)
PiZZ = 85/96; PiSZ = 8/96;
No further details
(covariates: treatment,
Other 3/96
provided
individual patient)
Tobacco exposure
the
Exsmokers = 70/96
Never smoked = 12/96
Smoker = 14/96
under
BMI (kg/m2), mean (SD)
(CTH) Care.
22.9 (3.6)
FEV1 (L/s)
1.43 (0.65)
1982 Aged
FEV1 predicted %
released
41 (17.3)
Act
Abbreviations:
AE = adverse events,
ANCOVA = Analysis of covariance,
BMI = body mass index,
C = comparator group,
Comb = combined,
and
DLco = difusing capacity for carbon monoxide,
FEV1 = forced
expiratory volume in one second,
FRC = functional residual capacity,
FVC = forced vital capacity,
GLMM = generalised linear mixed model,
I = intervention group,
KCO = DLCO divided by alveolar volume,
PD15 =
15th percentile point,
RCT = randomised controlled trial,
SGRQ = St George’s Respiratory Questionnaire, SE =
TEAE = Treatment-emergent adverse events,
TLC = total lung capacity.
been
** Significant dif erence between groups p < 0.001.
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
164
Page 178 of 218
FOI 5155 - Document 4
Table 104
Characteristics of single arm studies used in the systematic literature review to assess safety
Authors
Design
Location
Baseline population characteristics Description of
Safety outcomes
Year
Evidence Length of
patient group/s
assessed
Study ID
level
follow-up
Intervention
Sample size
McElvaney Non-RCT, Ireland
Age
Intervention/Patients • Adverse events
et al. 2017 prospective 24 months
[I] = 53.8 ± 6.9
Early-start [48m]
• Severe adverse
RAPID-OLE III-2
N=140
[C] = 52.4 ± 7.8
Intravenous
Zemaira
events
FEV1 Predicted (%)
60mg/kg per week for • Treatment-related
[I] = 47.4 ± 12.1
48 months
adverse events
[C] = 47.2 ± 11.1
• Dyspnoea
Alpha-1 Antitrypsin (μM)
Delayed-start [24m]
[I] = 6.38 ± 4.62
Intravenous
Zemaira
[C] = 5.94 ± 2.42
60mg/kg per week for the
24 months
Lung density (g/L)
TLC-[I] = 45.5 ± 15.8
Early-start [48m] = 76
TLC-[C] = 48.9 ± 15.5
Delayed-start [24m] =
under
FRC-[I] = 47.6 ± 15.7
64
(CTH) Care.
FRC-[C] =50.7 ± 15.0
Comb-[I] = 46.6 ± 15.6
Comb-[C] = 49.8 ± 15.1
1982 Aged
Genotype (both arms)
PiZZ = 168 (93%)
released
Act
The Alpha- 37-site
USA
Age
Intervention =
and • Discontinuation due
1-Antitrypsin Observation 5 years
46 ± 11
Prolastin
to adverse event
Deficiency al cohort
N=927
Gender (M:F) been
Patients = Registry
Registry
study
participants never
Study Group
(not all treated 510:417
(Registry)
(227), partly (261),
Health
1998
with
Tobacco exposure (both)
has
IV
intervention)
and always (389)
Nonsmokers = 198/927 of
receiving AT
Exsmokers = 655/927
Information
Current smokers = 75/927
of
FEV1 Predicted (%)
49 ± 30
Alpha-1 Antitrypsin (μM)
document
5.7
± 1.4
Barker et al. Retrospectiv USA
Age
Department
Intervention =
• Any adverse events
This
1994
e case
48 months
50 ± 6
Freedom
Prolastin
• Hospitalisation due
series
N=14
Gender (M:F)
Patients = NHLBI
to adverse event
the
IV
10:4
Registry for severe
• Discontinuation due
by
Tobacco exposure
AAT deficiency
to adverse event
Nonsmokers = 1
participants 14
• Death due to
Exsmokers = 13
adverse event
FEV
1-2 weekly Prolastin
1 Predicted (%)
infusions of 60mg/kg
0.9 ± 0.4
AAT mg/dl
41 ± 8.8
Phenotype PiZ
Barker et al. Open label, USA
Age
Intervention = A1PI
• Any adverse events
1997
uncontrolled 4 months
51.1 ± 7.2
Patients = 23
• Severe adverse
pharmacoki
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
165
Page 179 of 218
FOI 5155 - Document 4
Authors
Design
Location
Baseline population characteristics Description of
Safety outcomes
Year
Evidence Length of
patient group/s
assessed
Study ID
level
follow-up
Intervention
Sample size
netic study N=23
Pulmonary impairment
events
IV
Severe 18/23
2 weekly A1PI
• Treatment-related
Other 5/23
infusions of 120
adverse events
AAT mg/dl
mg/kg for 10 infusions • Dyspnoea
≤50
• Death due to
Phenotype
adverse event
PiZ
Barros-Tizón Multicentre, Spain
Age
Intervention =
• Any adverse events
et al.
retrospectiv 18 months
51.7 ± 9.1
Prolastin or
Trypsone • Severe adverse
2012
e case
N=27
FEV1 predicted
Patients = 27
events
the
series study
46.0 ± 13.4
• Treatment-related
IV
AAT serum concentrations
1-3 weekly A1PI
adverse events
<11 µml (inclusion criteria)
infusions of 60mg/kg • Discontinuation due
Tobacco exposure
Prolastin/
Trypsone
to adverse event
under
(CTH) Care.
Smokers 4/127 (3%)
• Death due to
Exsmokers 100/127 (94%)
adverse event
Nonsmokers 22/127 (17%)
Genotype
1982 Aged
PiZZ 118/127 (96%)
released
PiSZ 1/127 (0.8%)
Act and
Other 7/127 (6%)
Campos et Multicentre, USA
Age
Intervention/Patients •
been
Any adverse events
al. 2013
double
4 months
[60] 59.7 ± 6.7
60mg/kg
• Severe adverse
blind, cross- N=30
[120] 57.4 ± 6.3
PROLASTIN-C = 15
events
Health
over study
has
FEV1 predicted
120 mg/kg
• Treatment-related
III-3
of
<80% both
PROLASTIN-C = 15
adverse events
Information
Phenotype
• Discontinuation due
[60] 13/15 P
of iZZ, 1/15 null, 1/15 PiZM 60mg/kg group
to adverse event
[120] all PiZZ
Weekly
PROLASTIN- • Death due to
C infusions of
adverse event
document
60mg/kg for 8 weeks
120 mg/kg group
Department
Patients crossed-over
This
to the other group for
Freedom
a further 8 weeks
the
(swapped)
Hubbard & Case series, USA
Age
Intervention =A1PI
•
by
Adverse events
Crystal1988 propsective 12 months
46 ± 8.0
Patients = 9
• Changes in body
IV
N=9
Gender (M:F)
weight,
6:3
A1PI Infusions of 250
abnormalities in
Phenotype
mg/kg every 28 days
blood or urine,
PiZZ = 8/9
antibodies against
AAT
PiZ null = 1/9
• Infection from
Tobacco exposure
treatment
Nonsmokers = 6/9
Exsmokers = 3/9
AAT serum levels
35 ± 10 mg/dL (4.7 µmol)
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
166
Page 180 of 218
FOI 5155 - Document 4
Authors
Design
Location
Baseline population characteristics Description of
Safety outcomes
Year
Evidence Length of
patient group/s
assessed
Study ID
level
follow-up
Intervention
Sample size
FEV1 predicted
78% ± 17%
Sandhaus et RCT
USA
Age
Intervention/Patients • Adverse events
al. 2014
Open label 3-site multi
[G] = 55.4 ± 7.7
Glassia =
33
occurring in >10 %
extension centre, first 3 [P] = 55.7 ± 9.2
Prolastin = 17
patients
III-2
months
Gender (M:F)
• Severe adverse
randomised
events
followed by
25:25
GLASSIA
• Treatment-related
open label
Race
Intravenous A1PI
adverse events
Caucasian = 49
60mg/kg
• Discontinuation due
the
7 months
Hispanic = 1
PROLASTIN
to adverse event
N=50
Phenotype
Intravenous A1PI
PiZZ = 43
60mg/kg
PiMZ = 2
under
PiSZ = 2
(CTH) Care.
Unknown = 3
FEV1 predicted
[G] 46 ± 17
1982 Aged
[P] 47 ± 23
released
Alpha-1 Antitrypsin (μM)
Act
[G] 4.8 ± 2
and
[P] 4.3 ± 1
been
Schmidt et Case series, Germany
Age
Intervention = A1PI • Any adverse events
al. 1988
multicentre, 6 months
46.5 ± 7.6
Patients = 20
• Severe adverse
unknown if
Health
N=20
Gender (M:F)
events
has
prospective
15:5
Weekly A1PI infusions
of
IV
Phenotype
of 60mg/kg for 6
Information
PiZ = 20/20
months
of
Tobacco exposure
Nonsmokers = 5/20
Exsmok
document ers = 15/20
FEV1 one second
1,094 ± 319
This Freedom
Department
Schwaiblmai Case series, Germany
Age
Intervention = A1PI
• Adverse events
r et al. 1997 prospective 36 months
48 ±
the 1.8
Patients = 20
• Hospitalisation due
IV
N=20
Gender (M:F)
to adverse event
by 11:9
Weekly A1PI infusions • Severe adverse
Phenotype
of 60mg/kg for 36
events
PiZZ = 19/20
months
• Treatment-related
PiSZ = 1/20
adverse events
Tobacco exposure
• Death due to
Nonskomers = 3/20
adverse event
Exsmokers = 17/20
AAT serum levels
43 ± 4.0 mg/dL
FEV1 predicted
41.7 ± 3.1
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
167
Page 181 of 218
FOI 5155 - Document 4
Authors
Design
Location
Baseline population characteristics Description of
Safety outcomes
Year
Evidence Length of
patient group/s
assessed
Study ID
level
follow-up
Intervention
Sample size
Stocks et al. Multicentre, USA
Age
Prolastin = 12
• Any adverse events
2010
double
6 months
57.7 ± 8.04
PROLASTIN-C = 12 • Infection from
blind, cross- N=24
Gender (M:F)
treatment
over study
14:10
Weekly infusions of
• Severe adverse
III-3
Phenotype
60mg/kg for 6 months events
PiZZ = 23/24
• Treatment-related
PiSZ = 1/24
adverse events
AAT serum levels
• Discontinuation due
18.7 ± (3.9)
to adverse event
FEV1 predicted
• Death due to
the
43% ± 13.3
adverse event
Stoller et al. Observation USA
Age
Intervention = A1PI
• Any adverse events
2003
al cohort
7 years
Sometimes 47 ± 10
Patients
• Severe adverse
study,
under
N=747
Always 48 ± 9.0
Registry participants:
events
(CTH) Care.
prospective
(Registry)
Gender (M:F)
Sometimes receiving • Dyspnoea
Prolastin AT = 357
IV
Sometimes 204:153
• Discontinuation due
Always 226:164
Always receiving
to adverse event
1982 Aged
Tobacco exposure (both)
Prolastin AT = 390
• Hospitalisation due
Nonsmokers = 92/747
to adverse event
released
Exsmokers = 595/747
• Physician visit or
Act and
Current smokers = 60/747
new medication
FEV
due to adverse
1 Predicted (%)
been
event
Sometimes 0.37 ± 0.2
Always 0.37 ± 0.2
has
Health
Alpha-1 Antitrypsin (μM) of
Sometimes 5.7 ± 1.3
Information
Always 5.7 ± 1.3
Wencker et Case series, Germany
Age of
Intervention
=
• Any adverse events
al. 1998
prospective 6 years
47 ± 9.0
Prolastin
• Severe adverse
IV
N=443
Gender (M:F)
Patients = 443
events
document
292:151
• Hospitalisation due
Tobacco exposure
Weekly infusions of
to adverse event
This
Department
Exsmokers = 356
60mg/kg for 29
• Dyspnoea
Freedom
Nonsmokers = 87
months [Exsmokers]
the
and 23 months
• Discontinuation due
Phenotype
[Nonsmokers]
to adverse event
PiZZ = 394
• Death due to
by PiSZ = 31
adverse event
Null = 6
• Infection from
PiFZ = 3
treatment
Other/unknown = 9
FEV1 Predicted (%)
Ex 35.5 ± 14.8
Non 42.2 ± 18.2
Wewers et Case series USA
Age
Intervention = A1PI
• Any adverse events
al. 1987
with healthy 6 months
46 ± 8
Patients = 21
• Severe adverse
controls,
N=21
Gender (M:F)
events
unknown if
Weekly infusions of
• Infection from
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
168
Page 182 of 218
FOI 5155 - Document 4
Authors
Design
Location
Baseline population characteristics Description of
Safety outcomes
Year
Evidence Length of
patient group/s
assessed
Study ID
level
follow-up
Intervention
Sample size
prospective
18:3
60mg/kg for 6 months treatment
III-2
Tobacco exposure
Exmokers = 19
Nonsmokers = 2
Phenotype
PiZZ = 21/21
FEV1 Predicted (%)
37
± 3
Alpha-1 Antitrypsin (μM)
4.2 ± 0.8
the
Abbreviations:
AE = adverse event;
AT = augmentation therapy;
NHBLI = National Heart, Lung, and Blood Institute,
RCT = randomised
controlled trial.
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
169
Page 183 of 218
FOI 5155 - Document 4
APPENDIX D EVIDENCE PROFILE TABLES
Table 105
Evidence profile table of effectivness outcomes for A1PI compared to placebo for patients with severe AATD and emphysema
the
Outcome
No. of
Risk of
Inconsisten
Indirectne
Imprecisio Other
No. of
No. of
Relativ
Absolute
Quality
Importan
(units,
studies
bias
cy
ss
n
consideratio
patients in
patient
e effect effect
ce
follow-up)
and study
ns (e.g.
A1PI arm
s in
(95%CI) (95%CI)
design
publication
placeb
under
o Care.
bias)
arm
(CTH)
Mortality
1 RCT
not
not serious
not serious serious a
none
1/93 (1.1%) 3/87
RR 0.35 MD 22
⨁⨁⨁⨀
Critical
(follow up:
serious
(3.4%)
(0.05 to fewer per
Moderate
24 months)
2.27)
1,000
1982 Aged
(from 33
released
fewer to 44
Act and
more)
Mortality
2
serious
serious b
not serious serious a
none
87/774
25/317
not
see
⨁⨀⨀⨀
Critical
(follow up:
Observation d,e,f
been
(11.2%)
(7.9%)
pooled
comment
Very low
median 47
al studies
months)
has
Health
SGRQ
2 RCTs
not
serious b
not serious serious a
none
128
120
-
MD 0.83
⨁⨁⨀⨀
Critical
of
Score (follow
serious
points lower Low
up: range 24
Information
(3.49 lower
to 30
to 1.83
of
months;
higher)
Scale from:
0 to 100)
document
Quality of life 2
serious
not serious
not serious serious a
none
Increased
⨁⨀⨀
Critical
Quality of
2
serious d,e,f
(follow up:
Observation d,e,f
quality of life ⨀
life (follow
Observation
Department
12 months;
al studies g
was
This
Very
up: 12
al studies g
Freedom
assessed
observed in low
months;
with: SGRQ
the
3/5
assessed
score or
measures.
with: SGRQ
narrative)
by
score or
narrative)
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
170
Page 184 of 218
FOI 5155 - Document 4
Annual
2 RCTs
not
not serious
not serious serious a
none
Higher reported RR (1.26, 95% CI 0.92 to 1.74),
⨁⨁⨁⨀
Critical
exacerbation
serious
MD (0.36, 95% CI -0.44 to 1.16) in A1PI group;
Moderate
rate (follow
these dif erences were not significant
up: range 24
to 30
months)
the
Number of
1
serious e,f not serious
not serious serious a,h
publication
The mean
⨁⨀⨀
Critical
Number of
1
serious e,f
exacerbation Observation
bias strongly
(SD)
⨀
exacerbatio
Observation
s (follow up:
al study
suspected i
number of
Very
ns (follow
al study
36 months)
exacerbatio
low
under
up: 36
Care.
ns
(CTH)
months)
decreased
from 1.2
(1.6) before
1982 Aged
AT to 1 (2.2)
after AT (
released p
< 0.01)
Act and
CT-
3 RCTs
not
not serious
not serious not serious none
155
148
-
MD 0.87 g/L ⨁⨁⨁⨁
Critical
measured
serious
higher
High
been
lung density
(0.31 higher
(follow up:
to 1.42
Health
range 24 to
has
higher)
30 months;
of
assessed
Information
with: g/mL)
Hospitalisati
of
2 RCTs
not
serious b
not serious serious
a
publication
Reported RR ranges from 0.56 (0.23 to 1.36) to
⨁⨀⨀⨀
Important
on due to
serious
bias
1.41 (0.57 to 3.48)
Very low
COPD
suspected c
document
exacerbation
(follow up:
range 24 to
This
Department
30 months)
Freedom
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
171
Page 185 of 218
FOI 5155 - Document 4
Change in
3 RCTs
not
not serious
not serious serious a
none
159
154
-
SMD 0.17
⨁⨁⨁⨀
Important
FEV1 (mL or
serious
SD lower
Moderate
% predicted)
(0.4 lower to
(assessed
0.05 higher)
with:
spirometry)
the
Change in
4
serious
serious b
not serious serious a
none
1068
510
-
see
⨁⨀⨀⨀
Important
FEV1 (mL or Observation d,e,f
comment
Very low
% predicted) al studies g
(follow up:
under Care.
median 52
(CTH)
months;
assessed
with:
1982 Aged
spirometry)
released
Carbon
3 RCTs
randomis
not serious
not serious not serious serious a
155
151
-
SMD 0.11
⨁⨁⨁⨀
Important
Act
monoxide
ed trials
and
SD lower
Moderate
dif usion
(0.34 lower
(DLCO)
to 0.11
been
(follow up:
higher)
range 24 to
Health
30 months;
has
assessed
of
with:
Information
mmol/min/kP
a or mL/mm
of
Hg per min;
%)
document
Carbon
1
serious b,c not serious
not serious serious a,e
publication
127
127
-
MD 10.2
⨁⨀⨀⨀
Important
monoxide
Observation
bias strongly
(23.1 lower
Very low
dif usion
al study
suspec
Department ted d
to 2.7
(D
This Freedom
LCO)
higher)
(follow up:
the
range 24 to
30 months;
by
assessed
with: %)
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
172
Page 186 of 218
FOI 5155 - Document 4
Lung
1
serious d,f not serious
not serious serious a
none
Lung
⨁⨀⨀
Importa
infection
Observation
infection < 2 ⨀
nt
(follow up:
al study g
increased
Very
range 1
from 27/89
low
years to 10
to 73/89
years;
Lung
the
assessed
infection ≥ 2
with: Self-
decreased
reporting)
from 62/89
to 16/89 under
Care.
Hospitalisati
2
serious
(CTH)
e,f
not serious
not serious serious a,j
publication
The
⨁⨀⨀
Importa
on (follow
Observation
bias strongly
average
⨀
nt
up: median
al studies g
suspected i
time spent
Very
24 months;
in hospital
low
1982 Aged
assessed
decreased
with:
on average
released
Number of
by a day
Act and
days spent
in hospital)
been
Abbreviations:
CI = Confidence interval,
RR = Risk ratio,
MD = Mean dif erence,
SMD = Standardised mean dif erence.
a. Underpowered to detect dif erences, b. Contradictory results not explained by sensitivity analysis, c. Exacerbations were recorded but not reported in at least one additional trial, d. Baseline population
dif erences, e. Patients excluded from analysis without clear explanations, f. Poorly defined intervention and comparators, g. Before and after studies and cohort studies, h. Fulfils the MCID for exacerbations but the
has
Health
reduction is very small (1.2 to 1), i. Missing data, j. No or poor reporting of statistical analysis
GRADE Working Group grades of evidence (Guyatt et al., 2013)
of
⨁⨁⨁⨁
High quality: We are very confident that the true ef ect lies close to that of the estimate of effect.
Information
⨁⨁⨁⨀
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially dif erent.
⨁⨁⨀⨀
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially dif erent from the estimate of the effect.
of
⨁⨀⨀⨀
Very low quality: We have very lit le confidence in the effect estimate: The true effect is likely to be substantially dif erent from the estimate of effect.
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
173
Page 187 of 218
FOI 5155 - Document 4
Table 106
Evidence profile table of safety outcomes for A1PI compared to placebo for patients with severe AATD and emphysema
Outcome
No. of
Risk of
Inconsiste Indirectness Imprecision Other
Effect
Quality
Importance
No. patients
studies and
bias
-ncy
considerations
Follow-up
study design
(publication
(median,
bias)
the
(range))
Severe AE
2 RCTs
not
not serious not serious
not serious
none
37/131
43/126
RR 0.83 58 fewer
⨁⨁⨁⨁ Critical
No. of patients:
serious
(28.2%)
(34.1%) (0.57 to
per 1,000
High
257
1.19)
(from 65
under
F/U: 30 months
more to 147
(CTH) Care.
(24 - 36 months)
fewer)
Severe AE
11
serious a
not serious not serious
not serious
none
Median occurrence 2.1% (0.0-30.0%)
⨁⨀⨀⨀ Critical
No. of patients:
Observational
Very low
1982 Aged
F/U: 6 months (3 studies
released
– 84 months))
Act and
Death due to AE
1 RCT
not
not serious not serious
serious b
none
No treatment-related deaths were reported in
⨁⨁⨁⨀ Critical
No. of patients:
serious
any of the included RCTs
Moderate
been
180
F/U: 24 months
(NA)
has
Health
Death due to AE
7
serious a
not serious not serious
not serious
none
Median occurrence 0.0% (0.0-7.1%)
of
⨁⨀⨀⨀ Critical
No. of patients:
Observational
Very low
Information
F/U: 18 months
studies
of
(4 – 72 months)
Discontinuation
2 RCTs
not
not serious not serious
serious b
none
1/131
6/126
RR 0.22 37 fewer
⨁⨁⨁⨀ Critical
due to AE
serious
document
(0.8%)
(4.8%)
(0.04 to
per 1,000
Moderate
No. of patients:
1.30)
(from 14
257
more to 46
Department
F/U: 27 months
fewer)
This Freedom
(24 – 30 months)
the
Discontinuation
8
not
not serious not serious
not serious
none
Median occurrence 0.6% (0.0-7.1%)
⨁⨁⨀⨀ Critical
due to AE
Observational serious
Low
by
No. of patients:
studies
F/U: 33 months
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
174
Page 188 of 218
FOI 5155 - Document 4
Outcome
No. of
Risk of
Inconsiste Indirectness Imprecision Other
Effect
Quality
Importance
No. patients
studies and
bias
-ncy
considerations
Follow-up
study design
(publication
(median,
bias)
(range))
(3 – 84 months)
the
Hospitalisation
4
not
not serious not serious
not serious
none
Median occurrence 1.4% (0.0-14.3%)
⨁⨁⨀⨀ Important
due to AE
Observational serious
Low
No. of patients:
studies
under
F/U: 60 months
(CTH) Care.
(36 – 84 months)
Any adverse
3
not
serious c
not serious
not serious
none
129/159
124/154
RR 1.00 0 fewer per ⨁⨁⨁⨀ Important
events
RCTs
serious
(81.1%)
(80.5%) (0.97 to
1,000
Moderate
1982 Aged
No. of patients:
1.03)
(from 24
313
released
fewer to 24
F/U: 30 months
more)
Act and
(24 – 36 months)
been
Any adverse
13
serious a
not serious not serious
not serious
none
Median occurrence 24.2% (0.0-100%)
⨁⨀⨀⨀ Important
events
Observational
Very low
Health
has
No. of patients:
studies
of
1747
Information
F/U: 12 months
(3 – 84 months)
of
Treatment-
2
not
not serious not serious
not serious
none
32/131
36/126
RR 0.86 40 fewer
⨁⨁⨁⨁ Important
document
related adverse
RCTs
serious
(24.4%)
(28.6%) (0.57 to
per 1,000
High
events
1.29)
(from 83
No. of patients:
Department
more to 123
This
257
fewer)
Freedom
F/U: 27 months
the
(24 – 30 months)
by
Treatment-
7
serious a
not serious not serious
not serious
none
Median occurrence 10.0% (0.0-28.6%)
⨁⨀⨀⨀ Important
related adverse
Observational
Very low
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
175
Page 189 of 218
FOI 5155 - Document 4
Outcome
No. of
Risk of
Inconsiste Indirectness Imprecision Other
Effect
Quality
Importance
No. patients
studies and
bias
-ncy
considerations
Follow-up
study design
(publication
(median,
bias)
(range))
events
studies
the
No. of patients:
F/U: 6 months (4
– 48 months)
under
Dyspnoea
2
not
serious c
not serious
serious b
none
17/131
13/126
RR 1.27 28 more
⨁⨁⨀⨀ Important
(CTH) Care.
No. of patients:
RCTs
serious
(13.0%)
(10.3%) (0.59 to
per 1,000
Low
257
2.71)
(from 42
F/U: 27 months
fewer to
(24 – 30 months)
176 more)
1982 Aged
released
Dyspnoea
4
not
not serious not serious
not serious
none Act Median occurrence 18.3% (3.8-34.8%)
and
⨁⨁⨀⨀ Important
No. of patients:
Observational serious
Low
F/U: 60 months
studies
been
(4 – 84 months)
has
Health
Abbreviations:
AE = adverse events,
CI = confidence interval;
F/U = follow-up,
RR = risk ratio.
of
a Limited length of follow-up to detect events, outcomes not clearly defined a priori
b Small sample size for rare event
Information
c Contradictory results across studies
GRADE Working Group grades of evidence (Guyatt et al., 2013)
of
⨁⨁⨁⨁
High quality: We are very confident that the true ef ect lies close to that of the estimate of effect.
⨁⨁⨁⨀
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially dif erent.
⨁⨁⨀⨀
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially dif erent from the estimate of the effect.
document
⨁⨀⨀⨀
Very low quality: We have very lit le confidence in the effect estimate: The true effect is likely to be substantially dif erent from the estimate of effect.
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
176
Page 190 of 218
FOI 5155 - Document 4
Table 107
Modified quality appraisal of included case series investigations according to the IHE Quality Appraisal of Case Series Studies (Guo et al. 2016)
to
f
?
d
ultiple
a
res
ng
ted?
udy
arly
s an
le
si
o
es o
at a es?
orted
tudy
linded
in m
c
ly
easu
u
est
gh t
epor
im at m
ep
ted?
ted
f the st
ted
istics
tudy at ease?
ear
e m
rs b
red
ts?
utco
ts r
inter
the
f the s
er
riteria
is
m
?
nou
lec
uited
act
n the d
y c
the s
de est
t o
tion cl
easu
t even
even
ons o lts?
ting t repor
ive o
ecr
ar
utco riori?
ong e
rovi
pe
?
conduc
ilit
ter the d
ility i
se
usi esu
y?
es co
ethods
evan
ts r y?
t ch
ib
t in
ven
t o
e assesso ion?
es m
to folow-up r
ver
ncl y r
om uppor
bject ed
lig
ts en
a p
m
m
portan
iab f rel
b
s
tat
tudy ivel
ien tivel
ien ?
oin
nter ?
evan ed
w-up l m
udy p
h c
ID
iate m
var
under
he o
at
at
ien
utco
utco
s o
ly s
he s ect
he cas
?
r p
el
ervent
osses
he ad
he co
e t
ibed
he i ibed
lish
tres?
e p
at
opr
ysi
ces of
(CTH) Care.
nsecu
e p
re the e ed
ila
e r ab
e o int
e o
s follo ture i
e l
dom al
e t
e t pported
Study
as t ear
as t
er
er
er
at
as t
er
er
er
er
er
er
ere bot
W cl
W prosp
W cen
W co
W descr
We st
Did p sim
W descr
W est
W the
W appr
Wa cap
W
Did the st ran an
W
W su
W sour
Alpha-1 Registry
Group (1998)
?
•
•
1982 Aged
Barros-Tizón et
al. (2012)
•
?
?
?
released
Barker et al.
Act and
(1994)
•
NA
•
Barker et al.
(1997)
•
•
been
Campos et al.
(2013)
?
•
•
Health
Hubbard &
has
Crystal (1988)
?
?
?
•
•
?
of
Sandhaus et al.
(2014)
•
Information
Schmidt et al.
of
(1988)
?
•
?
•
•
•
Schwaiblmair et
al. (1997)
?
?
?
•
?
•
?
•
document
Stocks et al.
(2010)
•
•
Stoller et al.
Department
(2003)
•
•
•
•
This Freedom
Wencker et al.
(1998)
•
•
the
Wewers et al.
(1987)
?
?
?
?
•
•
•
?
•
by
= Yes; = No; ? = unclear; • = partial.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
177
Page 191 of 218
FOI 5155 - Document 4
Table 108
Risk of bias in non–randomised studies comparing A1PI augmentation therapy and best supportive care or placebo
Study
Bias due to
Bias selection of
Bias in
Bias due to
Bias due to missing
Bias in
Bias in selection of
Overall Bias
reference/ID
confounding participants into the measurement of
departures from
data
measurement of the reported results
study
intervention
intended
outcomes
interventions
the
Alpha-1 registry
Serious
Moderate
Serious
Low
Serious
Moderate
Serious
Serious
group 1998
Barros-Tizon et
Serious
Moderate
Serious
Low
Serious
Moderate
Serious
Serious
al. 2012
under
Karl et al. 2017
Serious
Low
Moderate
Low
Low
Moderate
Care. Moderate
Serious
(CTH)
Lieberman 2000
Serious
Serious
Serious
Low
Low
Moderate
Low
Serious
McElvaney et al.
Low
Low
Low
Low
Low
Moderate14
Aged
Moderate15
Moderate
1982
2017
released
Seersholm et al.
Serious
Moderate
Low
Low
Low
Moderate
Low
Serious
Act and
1997
Tonelli et al.
Serious
Serious
Serious
Moderate
Serious
Moderate
Moderate
Serious
been
2009
Wencker et al.
Serious
Moderate
Serious
Low
Low
Moderate
Moderate
Serious
Health
1998
has
Information
of
of
document
This Freedom
Department
the
14 Assessors not blinded to intervention, outcome of all-cause mortality could be subject to negligible assessor judgement
by
15 Outcomes are consistent with an a priori plan, there is no indication of selection of reported patients of analyses
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
178
Page 192 of 218
FOI 5155 - Document 4
Table 109
Safety outcomes reported in RCT studies
Authors
Adverse events
Adverse events
Severe adverse events
Treatment-related
Discontinuation/
Publication Intervention
Control
adverse events
Hospitalisation /
Year
Mortality due to adverse
Study ID
events (state which)
the
Chapman et Events
Events
Patients
Patients
Events
Death due to an adverse
al. 2015
Total AE
1298
Total AE
1068
Intervention:
Control:
Intervention:
event
RAPID*
25/93 (27%)
27/87 (31%)
91
Intervention:
Patients
Patients
28/93 (30%)*
28/87 (32%)*
under
Control:
1 (1%) respiratory failure
Care.
Total AE
92 (99%)
Total AE
86 (99%)
(CTH) 50
Control:
Patients (n=93)
Patients (n=87)
3 (3.5%) sepsis,
Events
Events
Intervention:
Control:
Patients
pneumonia, breast cancer
Infections/Infestations 334
Infections/Infestations 369
COPD 9 (10%)
Pneumoni
1982 a 6 (5%)
Aged Intervention:
Respiratory disorders 249
Respiratory disorders 127
Pneumonia 3 (3%)
Lower respiratory 4 21 (23%)
Discontinuation due to an
released
Nervous system
194
Nervous system
134
Condit. aggravated 2 (5%)
Control:
adverse event
Act and
Admin site issues
144
Admin site issues
101
(2%)
Diverticulitis 2 (2%) 21 (24%)
Intervention:
COPD
107
COPD
53
Lung neoplasm 2 (2%) COPD 2 (2%)
1/93 (1%)
been
GI disorders
104
GI disorders
92
Dizziness 2 (2%)
Dyspnoea 2 (2%)
Control:
Headache
98
Headache
105
Pneumothorax 2 (2%) Palpitations 1 (1%)
4/87 (5%)
has
Health
Lower respiratory
88
Lower respiratory
72
Confusional state 1
Eye inflammation 1
of
MS disorders
68
MS disorders
75
(1%)
(1%)
Aggravation
62
Aggravation
41
Suicide attempt 1 (1%) Intestinal obstruction 1
Information
Nasopharyngitis
53
Nasopharyngitis
58
Hydronephrosis 1 (1%) (1%)
of
Oropharyngeal pain 36
Oropharyngeal pain 13
Angina 1 (1%)
Joint injury 1 (1%)
Cough
31
Cough
7
Abdominal pain 1 (1%) Cholelithiasis 1 (1%)
document
Dyspnoea
29
Dyspnoea
11
Back pain 1 (1%)
Bronchitis 1 (1%)
Bronchitis
26
Bronchitis
16
Bladder cancer 1 (1%) Appendicitis 1 (1%)
Department
Upper respiratory
26
Upper respiratory
25
Dyspnoea 1 (1%)
Cellulitis 1 (1%)
This Freedom
Nausea
23
Nausea
11
GORD 1 (1%)
Graft infection 1 (1%)
Sinusitis
17
Sinusitis the
25
Ileus 1 (1%)
Pyelonephritis 1 (1%)
Pneumonia
15
Pneumonia
18
Nausea 1 (1%)
Sepsis 1 (1%)
by
Pyrexia
15
Pyrexia
8
Small intestinal
Back pain 1 (1%)
Influenza
14
Influenza
12
obstruction 1 (1%)
Lung neoplasm 1 (1%)
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
179
Page 193 of 218
FOI 5155 - Document 4
Authors
Adverse events
Adverse events
Severe adverse events
Treatment-related
Discontinuation/
Publication Intervention
Control
adverse events
Hospitalisation /
Year
Mortality due to adverse
Study ID
events (state which)
Fatigue
14
Fatigue
12
Adhesion 1 (1%)
Muscle spasm 1 (1%)
Back pain
12
Back pain
12
Chest pain 1 (1%)
Chest pain 1 (1%)
the
Haemorrhage post
Breast cancer 1 (1%)
Patients (n=93)
Patients (n=87)
treatment 1 (1%)
Parathyroid tumour
Infections/Infestations 77 (83%)
Infections/Infestations 76 (87%)
Hyponatraemia 1 (1%) benign 1 (1%)
under
Respiratory disorders 63 (68%)
Respiratory disorders 49 (56%)
Influenza 1 (1%)
Syncope 1 (1%)
(CTH) Care.
Admin site issues
48 (52%)
Admin site issues
42 (48%)
Pyrexia 1 (1%)
Nephrolithiasis 1 (1%)
GI disorders
46 (49%)
GI disorders
47 (54%)
Anaphylactic
Pneumothorax 1 (1%)
Nervous system
46 (49%)
Nervous system
43 (49%)
reaction 1 (1%)
DVT 1 (1%)
1982 Aged
Headache
37 (40%)
Headache
33 (38%)
Lower respiratory 1
released
MS disorders
35 (38%)
MS disorders
37 (43%)
(1%)
Act
Nasopharyngitis
30 (32%)
Nasopharyngitis
26 (30%)
Diverticulitis 1 (1%)
and
COPD
30 (32%)
COPD
20 (23%)
Bronchitis 1 (1%)
Oropharyngeal pain 22 (24%)
Oropharyngeal pain 10 (11%)
Gastroenteritis viral 1
been
Cough
20 (22%)
Cough
7 (8%)
(1%)
Aggravation
20 (22%)
Aggravation
14 (16%)
UTI 1 (1%)
has
Health
Lower respiratory
18 (19%)
Lower respiratory
17 (20%)
Intervertebral disc
protrusion 1 (
of 1%)
Dyspnoea
17 (18%)
Dyspnoea
10 (11%)
Pulmonary em
Information bolism 1
Nausea
15 (16%)
Nausea
8 (9%)
(1%)
of
Influenza
14 (15%)
Influenza
10 (11%)
Respiratory failure 1
Upper respiratory
14 (15%)
Upper respiratory
14 (16%)
(1%)
Pyrexia
13 (14%)
Pyrexia
6 (
document 7%) Hypotension 1 (1%)
Bronchitis
12 (13%)
Bronchitis
11 (13%)
Sinusitis
12 (13%)
Sinusitis
10 (11%)
This
Department
Back pain
12 (13%)
Back pain
10 (11%)
Freedom
Pneumonia
11 (12%)
Pneumonia
12 (14%)
the
Fatigue
8 (9%)
Fatigue
10 (11%)
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
180
Page 194 of 218
FOI 5155 - Document 4
Authors
Adverse events
Adverse events
Severe adverse events
Treatment-related
Discontinuation/
Publication Intervention
Control
adverse events
Hospitalisation /
Year
Mortality due to adverse
Study ID
events (state which)
Dirksen et Adverse events
28
Adverse events
40
Patients
Events
Discontinuation due to an
al. 2009
the
Patients
37 (99%)
Patients
38 (99%)
Intervention:
Control:
Intervention:
adverse event
EXACTLE*
9/38 (24%)
15/39 (38%)
14
Intervention: 0
Patients (n=38)
Patients (n=39)
Control:
Control: 2
Severe exacerbations 5 (13%)
Severe exacerbations 6 (15%)
Patients (n=38)
Patients (n=39)
35
under
Pneumonia
3 (8%)
Pneumonia
4 (10%)
Pneumonia 3 (8%)
Pneumonia 4 (10%)
(CTH) Care.
Pneumothorax
2 (5%)
Pneumothorax
0 (0%)
Atrial fibrillation 2 (5%) Pulmonary embolism Patients
Atrial fibrillation
2 (5%)
Atrial fibrillation
0 (0%)
Pneumothorax 2 (5%) 2 (5%)
Intervention:
Biliary colic
1 (3%)
Biliary colic
0 (0%)
Constipation 1 (3%)
Abdominal pain 1
11 (29%)
1982 Aged
Constipation
1 (3%)
Constipation
0 (0%)
GORD 1 (3%)
(2%)
Control:
released
Epistaxis
1 (3%)
Epistaxis
0 (0%)
Biliary colic 1 (3%)
Intra-abdominal
15 (38%)
Act and
Gall bladder disorder 1 (3%)
Gall bladder disorder 1 (3%)
Malaria 1 (3%)
haemorrhage 1 (2%)
GO reflux
1 (3%)
GO reflux
0 (0%)
Upper limb fracture 1 Rectal haemorrhage
been
Malaria
1 (3%)
Malaria
0 (0%)
(3%)
1 (2%)
Menorrhagia
1 (3%)
Menorrhagia
0 (0%)
Gallbladder disorder 1 Nodule 1 (2%)
Health
Psoriasis
1 (3%)
Psoriasis
0 (0%)
(3%)
Gallbladder disorder
has
TIA
1 (3%)
TIA
0 (0%)
TIA 1 (3%)
1 (2%)
of
Upper limb fracture 1 (3%)
Upper limb fracture 0 (0%)
Menorrhagia 1 (3%)
Jaundice 1 (2%)
Information
Abdominal pain
0 (0%)
Abdominal pain
1 (3%)
Epixtaxis 1 (3%)
Appendicitis 1 (2%)
of
IA haemorrhage
0 (0%)
IA haemorrhage
1 (3%)
Psoriasis 1 (3%)
Sepsis 1 (2%)
Rectal haemorrhage 0 (0%)
Rectal haemorrhage 1 (3%)
Subcutaneous
Nodule
0 (0%)
Nodule
1 (
document 3%)
abscess
Cholestatic jaundice 0 (0%)
Cholestatic jaundice 1 (3%)
1 (2%)
Appendicitis
0 (0%)
Appendicitis
1 (3%)
UTI 1 (2%)
This
Department
Sepsis
0 (0%)
Sepsis
Freedom 1 (3%)
Arthralgia 1 (2%)
Subcutaneous
0 (0%)
Subcutaneous
1 (3%)
Osteoarthritis 1 (2%)
the
abscess
0 (0%)
abscess
1 (3%)
Breast cancer 1 (2%)
UTI
0 (0%)
UTI by
1 (3%)
COPD 1 (2%)
Arthralgia
0 (0%)
Arthralgia
1 (3%)
Dyspnoea 1 (2%)
Pleural effusion 1
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
181
Page 195 of 218
FOI 5155 - Document 4
Authors
Adverse events
Adverse events
Severe adverse events
Treatment-related
Discontinuation/
Publication Intervention
Control
adverse events
Hospitalisation /
Year
Mortality due to adverse
Study ID
events (state which)
Osteoarthritis
0 (0%)
Osteoarthritis
1 (3%)
(2%)
Breast cancer
0 (0%)
Breast cancer
1 (3%)
Pulmonary oedema
the
COPD
0 (0%)
COPD
1 (3%)
1 (2%)
Dyspnoea
0 (0%)
Dyspnoea
1 (3%)
Lichen sclerosus 1
Pleural effusion
0 (0%)
Pleural effusion
2 (5%)
(2%)
under
Pulmonary embolism 0 (0%)
Pulmonary embolism 1 (3%)
(CTH) Care.
Pulmonary oedema 0 (0%)
Pulmonary oedema 1 (3%)
Lichen sclerosus
Lichen sclerosus
1982 Aged
Dirksen et Patients
Patients
NR
NR
NR
NR
released
al. 1999
Adverse effects
0/28
Adverse effects
0/28
Act
DIRKSEN99
and
been
Abbreviations:
AE = adverse event,
COPD = chronic obstructive pulmonary disorders,
DVT = Deep vein thrombosis,
GI = gastrointestinal,
GO = gastro-oesophageal,
GORD = gastrointestinal reflux disease,
IA =
Intra-abdominal,
MS = musculoskeletal,
TIA = Transient ischaemic attack,
UTI = Urinary tract infection. *Some data were obtained from the outcomes tab on www.clinicaltrials.gov
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
182
Page 196 of 218
FOI 5155 - Document 4
Table 110
Safety outcomes reported in single arm studies
Authors
Adverse events*
Severe adverse events
Treatment-related adverse events
Discontinuation/ Hospitalisation /
Publication
Events
Death due to adverse events
Year
Patients (%)
Study ID
the
McElvaney et Adverse events
[48m] 773
Patients
Patients
al. 2017
[24m] 620
Early-start [48m] (n=76)
Early-start [48m] (n=76)
RAPID-OLE Patients
[48m] 76 (100%)
23 (30%)
11 (15%)
[24m] 64 (97%)
under Care.
Delayed-start [24m] (n=64)
Delayed-start [24m] (n=64)
(CTH)
Patients (n=140)
19 (30%)
7 (11%)
COPD
56 (40%)
Nasopharyngitis
40 (28%)
1982 Aged
Headache
28 (20%)
released
Condition aggravated
27 (19%)
Act and
Lower respiratory tract
infection
20 (14%)
been
Oropharyngeal pain
19 (13%)
Dyspnoea
18 (13%)
Health
Upper respiratory tract
has
infection
17 (12%)
of
Influenza
16 (11%)
Information
Back pain
15 (11%)
of
Cough
13 (9%)
Pneumonia
13 (9%)
Oral candidiasis
document
13 (9%)
Bronchitis
12 (8%)
Diarrhoea
Department
12 (8%)
This
Oedema peripheral
Freedom
12 (8%)
Nausea
11 (8%)
the
The Alpha-1- NR
NR
NR
NR
Discontinuation due to an adverse
by
Antitrypsin
event 137/1129 (12%)
Deficiency
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
183
Page 197 of 218
FOI 5155 - Document 4
Authors
Adverse events*
Severe adverse events
Treatment-related adverse events
Discontinuation/ Hospitalisation /
Publication
Events
Death due to adverse events
Year
Patients (%)
Study ID
Registry Study
Group 1998
the
Barker et al. Adverse events
10
NR
NR
Hospitalisation due to an adverse event
1994
Patients
4 (28%)
2/14 (14%)
Patients (n=14)
under
Discontinuation due to an adverse
(CTH) Care.
Back pain
2 (14%)
event 1/14 (7%)
Headache
4 (28%)
Death due to an adverse event 1/14
Shortness of breathe
4 (28%)
(7%)
1982 Aged
Barker et al. Adverse events
27
Patients (n=23)
Patients (n=23)
Death due to an adverse event
1997
Patients
21 (91%)
0
Dyspnoea 3 (
released 13%)
(bronchopneumonia) 1/23 (4%)
Patients (n=23)
Chest
Act tightness 1 (4%)
and
Headache
10 (43%)
Fatigue
9 (39%)
been
Dyspnoea
8 (35%)
Cough
2 (8%)
has
Health
Chest tightness
1 (4%)
of
Barros-Tizón Adverse events
14
Patients (n=127)
Patients (n=127)
Discontinuation due to an adverse
Information
et al.
Patients
11 (8%)
Pulmonary TE 1 (0.8%)
Chills 1 (0.8%)
events 0/27
2012
of
Patients (n=127)
Myeloid leukaemia 1 (0.8%)
Facial redness 1 (0.8%)
Death due to an adverse event 0/27
Pulmonary TE
1 (0.8%)
Acute MI 1 (0.8%)
Sensation of cold 1 (0.8%)
Myeloid leukaemia
1 (0.8%)
Haemorrhagic infarction 1 (0.8%)
Mild oedema 1 (0.8%)
document
Acute MI
1 (0.8%)
Cutaneous exantema 1 (0.8%)
Haemorrhagic infarction 1 (0.8%)
Fever 1 (0.8%)
Department
Chills
1 (0.8%)
This
Anxiety 1 (0.8%)
Freedom
Facial redness
1 (0.8%)
the
Sensation of cold
1 (0.8%)
Mild oedema
1 (0.8%)
by
Cutaneous exantema
1 (0.8%)
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
184
Page 198 of 218
FOI 5155 - Document 4
Authors
Adverse events*
Severe adverse events
Treatment-related adverse events
Discontinuation/ Hospitalisation /
Publication
Events
Death due to adverse events
Year
Patients (%)
Study ID
Fever
1 (0.8%)
Anxiety
1 (0.8%)
the
Sleep apnoea
1 (0.8%)
UTI
1 (0.8%)
Hypertension
1 (0.8%)
under
Pneumonia
1 (0.8%)
(CTH) Care.
Campos et al. Adverse events
[60] 69
Patients (n=30)
Events
Discontinuation due to an adverse
2013**
[120] 43
[60] 0
[60] 5
event 0/30
Patients
[60] 23 (77%)
[120] 0
[120] 1
Death due to an adverse event 0/30
1982 Aged
[120] 18 (60%)
Patients (n=30)
released
Patients (n=30)
[60] 3 (10%)
Act and
COPD exacerbation
[60] 7 (23%)
[120] 1 (3%)
[120] 5 (17%)
been
UTI
[60] 3 (10%0
[120] 0
Health
Contusion
[60] 1 (3%)
has
[120] 0
of
Thrombocytopenia
[60] 2 (7%)
Information
[120] 0
of
Proteinuria
[60] 2 (7%)
[120] 1 (3%)
Increased blood creatinine [60] 0
document
[120] 2 (3%)
Increased blood glucose [60] 0
This
Department
[120] 2 (3%) Freedom
Hubbard &
Patients (n=9)
NR
the
NR
NR
Crystal1988 Clinical adverse events 0
by
Change in body weight 0
Infection from treatment 0
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
185
Page 199 of 218
FOI 5155 - Document 4
Authors
Adverse events*
Severe adverse events
Treatment-related adverse events
Discontinuation/ Hospitalisation /
Publication
Events
Death due to adverse events
Year
Patients (%)
Study ID
Sandhaus et Adverse events at 3 m
39
Patients (n=33)
Patients
Discontinuation due to an adverse
al. 2014
the
Adverse events at 6 m
25
3 months
3 months
event
GLASSIA
Patients
49 (98%)
Glassia:
Glassia:
Patients
Patients (n=50)
COPD exacerbation 1 (3%)
Headache 3/33 (9%)
3 months
3 months
Endoscopic retrograde
Hypertension 1/33 (3%)
Glassia:
under
Cough
9 (18%)
cholangiopancreatography 1 (3%)
Pulmonary embolism 1/33 (3%)
(CTH) Care.
Upper respiratory infection 4 (12%)
Prolastin:
Prolastin:
Nasopharyngitis
0
Prolastin:
Headache 1/17 (6%)
Urticaria 1/17 (6%)
Rash Pharyngeal pain
0
Pulmonary emboli 1 (6%)
Hypertension 1/17 (6%)
1982 Aged
Headache
0
released
COPD exacerbation
7 (14%)
6 months
Act and
Productive cough
4 (8%)
Glassia:
Nausea
3 (6%)
Urticaria 1/21 (5%)
been
Fatigue
3 (6%)
Influenza symptoms 1/21 (5%)
Epistaxis
3 (6%)
Lowered platelet count 1/21 (5%)
Health
Urticaria
2 (4%)
has
Joint swelling 1/21 (5%)
Hypersensitivity
2 (4%)
Dizziness 1/21 (5%)
of
2 (4%)
Rash 1/21 (5%)
Information
6 months (n=21)
of
Cough
Upper respiratory infection 0
Nasopharyngitis
10 (47%)
document
Rash
6 (28%)
Headache
4 (19%)
This
Department
COPD exacerbation
0
Freedom
Productive cough
0
the
Nausea
0
Fatigue
0
by
Epistaxis
0
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
186
Page 200 of 218
FOI 5155 - Document 4
Authors
Adverse events*
Severe adverse events
Treatment-related adverse events
Discontinuation/ Hospitalisation /
Publication
Events
Death due to adverse events
Year
Patients (%)
Study ID
Urticaria
0
Hypersensitivity
1 (5%)
the
0
Schmidt et al. Adverse events
2
Events
NR
NR
1988
Patients
2 (10%)
0
under
Patients (n=20)
(CTH) Care.
Haematoma-like spots
1 (5%)
Weight loss
1 (5%)
Infection from treatment 0
1982 Aged
Schwaiblmair
Adverse events
1
Events
Events
Hospitalisation due to an adverse event
released
et al. 1997
Patients
1 (5%)
0
0
0/20
Act and
Patients (n=20)
Death due to an adverse event 1/20
Fever and exanthema
1 (5%)
been
Infection from treatment 0
Stocks et al. Adverse events
14
Events
Events
Discontinuation due to an adverse
has
Health
2010**
Patients –Intervention 1 11/12 (46%)
2
Pruritus 2
event 0/24
of
Patients –Intervention 2 9/12 (37%)
Death due to an adverse event 0/24
Information
Patients – open label
11/12 (46%)
Patients (n=24)
Events
Pruritus 1 (4%)
of
Upper respiratory tract
infection
4
document
UTI
3
Headache
3
Department
Rales
2 This Freedom
Arthralgia
2
Infection from treatment
the
0
Stoller et al. Adverse events
990
Events
NR
Events
by
2003
Patients
174 (20%)
Dyspnoea 61
Discontinuation due to an adverse
Events
Wheezing 14
event 8/720 (1%)
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
187
Page 201 of 218
FOI 5155 - Document 4
Authors
Adverse events*
Severe adverse events
Treatment-related adverse events
Discontinuation/ Hospitalisation /
Publication
Events
Death due to adverse events
Year
Patients (%)
Study ID
Headache
339
Hypotension 2
Hospitalisation due to an adverse event
Dizziness
121
the
12/720 (2%)
Fever
64
Physician visit or new medication due
Dyspnoea
61
to an adverse event 152/720 (21%)
Flushing
54
under
Fever
53
(CTH) Care.
Nausea
53
Chills
47
Rash
37
1982 Aged
Chest tightness
37
released
Anxiety
32
Act
Mild pain
31
and
Muscle cramps
27
Tachycardia
20
been
Chest pain
16
Wheezing
14
has
Health
Emesis
12
of
Hypotension
2
Information
Infection from treatment 0
of
Wencker et al. Adverse events
124
Events 5
NR
Patients
1998
Patients
65 (15%)
Patients (n=443)
Hospitalisation due to an adverse event
Events
Anaphylactic
document reaction, 4 (1%)
5/443 (1%)
Nausea/vomiting
21
Worsened congestive heart failure with
Discontinuation due to an adverse
Increased dyspnoea
19
respiratory failure,1 (0.2%)
event 3/443 (1%)
This
Department
Uticaria
18
Death due to an adverse event 0/443
Freedom
Fever
17
the
Fatigue
7
by
Anaphylactic reaction
4
Worsened congestive
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
188
Page 202 of 218
FOI 5155 - Document 4
Authors
Adverse events*
Severe adverse events
Treatment-related adverse events
Discontinuation/ Hospitalisation /
Publication
Events
Death due to adverse events
Year
Patients (%)
Study ID
heart failure with
respiratory failure
1
the
Infection from treatment 0
Wewers et al. Adverse events
4
Patients (n=21)
NR
NR
1987
Patients
4
(19%)
0
under
Patients (n=21)
(CTH) Care.
Fever
4 (19%)
Infection from treatment 0
Abbreviations:
TE = thromboembolism,
m = months. *Two single arm studies combined in this column only. **These studies report on the same patients at dif erent time points and intervention products.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
189
Page 203 of 218
FOI 5155 - Document 4
APPENDIX E
EXCLUDED STUDIES
Studies potential y considered eligible for inclusion based on the PICO criteria, but subsequently
excluded (due to containing duplicate information, in another language and not being a higher level
of evidence than that available in English, being unable to extract the data etc), are described in the
table below.
Trial ID
Grounds for
Details
Report
seeking exclusion
Browne et
Study
No safety data or other
Browne, RJ, Mannino, DM & Khoury, MJ, 1996.
al. 1996
design/outcomes
relevant outcomes were
Alpha 1-antitrypsin deficiency dea
the ths in the United
reported in this observational
States from 1979-1991. An analysis using multiple-
study
cause mortality data,
Chest, 110(1), pp. 78-83.
Campos et Study
No safety data or other
Campos, MA, Alazemi, S
under , Zhang, G, Wanner, A,
Care.
al. 2009
(CTH)
design/outcomes
relevant outcomes were
Salathe, M, Baier, H & Sandhaus, RA, 2009a.
reported in this single-arm trial, Exacerbations in subjects with alpha-1 antitrypsin
there was some resource
deficiency receiving augmentation therapy,
Respir
1982 Aged
utilisation data that turned out
Med, 103(10), pp. 1532-1539.
released
to be of no use to the
Act and
economics
Dirksen et
Study
No safety data or other
Dirksen, A, Fri s, M, Olesen, KP, Skovgaard, LT &
been
al. 1997
design/outcomes
relevant outcomes were
Sorensen, K, 1997. Progress of emphysema in
reported in this observational
severe alpha 1
Health -antitrypsin deficiency as assessed
has
study
by annual CT,
Acta Radiol, 38(5), pp. 826-832.
of
Esquinas
Study
No safety data or other
Information Esquinas, C, Serreri, S, Barrecheguren, M,
et al. 2018
design/outcomes
relevant outcomes were
Rodriguez, E, Nunez, A, Casas-Maldonado, F,
of
reported in this observational
Blanco, I, Pirina, P, Lara, B & Miravitl es, M, 2018.
study
Long-term evolution of lung function in individuals
document
with alpha-1 antitrypsin deficiency from the Spanish
registry (REDAAT),
International Journal of COPD,
This
Department
13pp. 1001-1007.
Freedom
Green et
Study
the No safety data or other
Green, CE, Parr, DG, Edgar, RG, Stockley, RA &
al. 2016
design/outcomes
relevant outcomes were
Turner, AM, 2016. Lung density associates with
by
reported in this observational
survival in alpha 1 antitrypsin deficient patients,
study
Respir Med, 112pp. 81-87.
Hutsebaut
Study
No safety data or other
Hutsebaut, J, Janssens, W, Louis, R, Wil ersinn, F,
et al. 2015
design/outcomes
relevant outcomes were
Stephenne, X, Sokal, E & Derom, E, 2015. Activity
reported in this observational
of the alpha-1 antitrypsin deficiency registry in
study
Belgium,
COPD 12pp. 10-14.
King et al.
Study
No appropriate outcomes were King, MB, Campbel , EJ, Gray, BH & Hertz, MI,
1994
design/outcomes
reported in this biomarker
1994. The proteinase-antiproteinase balance in
study, which was not patient-
alpha-1-proteinase inhibitor-deficient lung
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
190
Page 204 of 218
FOI 5155 - Document 4
Trial ID
Grounds for
Details
Report
seeking exclusion
specific
transplant recipients,
Am J Respir Crit Care Med,
149(4), pp. 966-971.
Lara et al.
Study
No safety data or other
Lara, B & Miravitl es, M, 2015. Spanish Registry of
2015
design/outcomes
relevant outcomes were
Patients With Alpha-1 Antitrypsin Deficiency;
reported in this observational
Comparison of the Characteristics of PISZ and
study (registry)
PIZZ Individuals,
Copd, 12pp. 27-31.
Lara et al.
Study
No safety data or other
Lara, B, Blanco, I, Martinez, MT, Rodriguez, E,
2017
design/outcomes
relevant outcomes were
Bustamante, A, Casas, F, Cadenas, S, Hernandez,
reported in this observational
JM, Lazaro, L, Torres, M, Curi, S, Esquinas, C,
study (registry)
Dasi, F, Escribano, A, Herrero, I, Martinez-
the
Delgado, B, Michel, FJ, Rodriguez-Frias, F &
Miravitl es, M, 2017. Spanish Registry of Patients
With Alpha-1 Antitrypsin Deficiency: Database
Evaluation and Population A
under nalysis,
Arch
(CTH) Care.
Bronconeumol, 53(1), pp. 13-18.
Lara et al.
Study
No safety data or other
Lara, B, de la Roza, C, Vila, S, Vidal, R &
2007
design/outcomes
relevant outcomes were
Miravitl es, M, 2007. Development and results of
1982 Aged
reported in this observational
the Spanish registry of patients with alpha-1-
released
study (registry)
antitrypsin deficiency,
Int J Chron Obstruct Pulmon
Act and
Dis, 2(3), pp. 393-398.
Ma et al.
Study
No safety data or other
been Ma, S, Lin, YY, Cantor, JO, Chapman, KR,
2017
design/outcomes
relevant outcomes were
Sandhaus, RA, Fries, M, Edelman, JM, McElvaney,
Health
reported in this
has post-hoc
G & Turino, GM, 2017. The Ef ect of Alpha-1
analysis of biomarkers of
Proteinase Inhibitor on Biomarkers of Elastin
Information Degradation in Alpha-1 Antitrypsin Deficiency: An
Analysis of the RAPID/RAPID Extension Trials,
of
Chronic Obstructive Pulmonary Diseases, 4(1), pp.
34-44.
document
Miravitlles
Study
No safety data or other
Miravitlles, M, Vidal, R, Barros-Tizon, JC,
et al. 1997
design/outcomes
relevant outcomes were
Department
Bustamante, A, Espana, PP, Casas, F, Martinez,
This Freedom reported in this observational MT, Escudero, C & Jardi, R, 1998. Usefulness of a
the study (registry)
national registry of alpha-1-antitrypsin deficiency.
The Spanish experience,
Respir Med, 92(10), pp.
by
1181-1187.
Piitulainen
Study
No safety data or other
Pi tulainen, E, Bernspang, E, Bjorkman, S &
et al. 2003
design/outcomes
relevant outcomes were
Berntorp, E, 2003. Tailored pharmacokinetic
reported in this observational
dosing allows self-administration and reduces the
study
cost of IV augmentation therapy with human
alpha(1)-antitrypsin,
Eur J Clin Pharmacol, 59(2),
pp. 151-156.
Schluchter Study
No safety data or other
Schluchter, MD, Stol er, JK, Barker, AF, Buist, AS,
relevant outcomes were
Crystal, RG, Donohue, JF, Fallat, RJ, Turino, GM,
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
191
Page 205 of 218
FOI 5155 - Document 4
Trial ID
Grounds for
Details
Report
seeking exclusion
et al. 2000
design/outcomes
reported in this observational
Vreim, CE & Wu, MC, 2000. Feasibility of a clinical
study
trial of augmentation therapy for alpha(1)-
antitrypsin deficiency. The Alpha 1-Antitrypsin
Deficiency Registry Study Group,
Am J Respir Crit
Care Med, 161(3), pp. 796-801.
Schmid et
Study
No safety data or other
Schmid, ST, Koepke, J, Dresel, M, Hattesohl, A,
al. 2012
design/outcomes
relevant outcomes were
Frenzel, E, Perez, J, Lomas, DA, Miranda, E,
reported in this observational
Greulich, T, Noeske, S, Wencker, M, Teschler, H,
study of biomarkers
Vogelmeier, C, Janciauskiene, S & Koczulla, AR,
2012. The effects of weekly augmentation therapy
in patients with PiZZ alpha1-anti
the trypsin deficiency,
Int J Chron Obstruct Pulmon Dis, 7pp. 687-696
Stockley et Study
This review combined the
Stockley, RA, Parr, DG, Piitulainen, E, Stolk, J,
al. 2010
design/outcomes
results of EXACTLE and
Stoel, BC & Dirksen, A, 20
under 10. Therapeutic efficacy
(CTH) Care.
DIRKSEN99, no novel
of alpha-1 antitrypsin augmentation therapy on the
outcomes were presented
loss of lung tissue: an integrated analysis of two
randomised clinical trials using computed
1982 Aged
tomography densitometry,
Respir Res, 11pp. 136.
released
Stockley et Study
No safety data,or other
Stockley, RA, Bayley, DL, Unsal, I & Dowson, LJ,
Act and
al. 2002
design/outcomes
relevant outcomes were
2002. The effect of augmentation therapy on
reported in this observational
bronchial inflammation in alpha1-antitrypsin
been
study of biomarkers
deficiency,
Am J Respir Crit Care Med, 165(11),
pp. 1494-1498.
Health
has
Stoller et
Study
No safety data or other of
Stoller, JK, Brantly, M, Fleming, LE, Bean, JA &
al. 2000
design/outcomes
relevant outcomes were
Information Walsh, J, 2000. Formation and current results of a
reported in this observational
patient-organized registry for alpha(1)-antitrypsin
of
study (registry)
deficiency,
Chest, 118(3), pp. 843-848.
Ulmer et al. Study
No saf
document ety data or other
Ulmer, WT, Schmidt, EW & Rasche, B, 1990. Long
1990
design/outcomes
relevant outcomes were
term effect on lung function of alpha 1-protease
reported in this observat
Department ional inhibitor substitution therapy in COPD patients with
This Freedom study
Pi ZZ phenotype,
Eur Respir J Suppl, 9pp. 21s-
the
22s.
Zamora et
Study by
No safety data were reported
Zamora, NP, Pla, RV, Del Rio, PG, Margaleff, RJ,
al. 2008
design/outcomes
in this observational study
Frias, FR & Ronsano, JBM, 2008. Intravenous
human plasma-derived augmentation therapy in
alpha1-antitrypsin deficiency: From
pharmacokinetic analysis to individualizing therapy,
Annals of Pharmacotherapy, 42(5), pp. 640-646.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
192
Page 206 of 218
FOI 5155 - Document 4
REFERENCES
ABS 2016, 3412.0—Migration, Australia, 2015-16. 2071.0—Census of Population and Housing:
Reflecting Australia - Stories from the Census, viewed 7 Aug 2018, <
http://www.abs.gov.au/ausstats/abs@.nsf/mf/2071.0>.
Abboud, RT, Ford, GT & Chapman, KR, 2001. Alpha1-antitrypsin deficiency: a position statement of
the Canadian Thoracic Society, Can Respir J, 8(2), pp. 81-88.
Abramowicz, M (ed), 1988. Alpha1-proteinase inhibitor for alpha1-antitrypsin deficiency, Med Lett
Drugs Ther, 30(761), pp. 29-30.
ABS, 2016. Life Tables, States, Territories and Australia, 2013-2015, Australian Bureau of Statistics,
Canberra.
the
Al, MJ, Koopmanschap, MA, van Enckevort, PJ, Geertsma, A, van der Bij, W, de Boer, WJ &
TenVergert, EM, 1998. Cost-effectiveness of lung transplantation in The Netherlands: a
scenario analysis, Chest, 113(1), pp. 124-130
under Care.
Alkins, SA & O’Malley P, 2000, Should health-care systems pay for replacement therap
(CTH) y in patients
with alpha (1)-antitrypsin deficiency? A critical review and cost-effectiveness analysis. Chest,
117(3), pp. 875–880.
1982 Aged
Alpha 1 Foundation 2015, Alpha-1 antitrypsin deficiency healthcare provider's guide, Coral Gables,
released
FL, viewed
Act
<https://www.alpha1.org/Portals/0/Documents/HealthcareProviders
and Brochure.pdf>.
Alpha-1-Antitrypsin Deficiency Registry Study Group, 1998, Survival and FEV1 decline in individuals
been
with severe deficiency of alpha1-antitrypsin. The Alpha-1-Antitrypsin Deficiency Registry Study
Group, Am J Respir Crit Care Med, 158(1), pp. 49-59.
has
Health
American Thoracic and European Respiratory Society, 2003. American Thoracic Society/European
of
Respiratory Society statement: standards for the diagnosis and management of individuals
Information
with alpha-1 antitrypsin deficiency, Am J Respir Crit Care Med, 168(7), pp. 818-900.
of
American Thoracic Society, 1999. Dyspnea. Mechanisms, assessment, and management: a consensus
statement. American Thoracic Society, Am J Respir Crit Care Med, 159(1), pp. 321-340.
document
Anthonisen, NR, Connett, JE, Kiley, JP, Altose, MD, Bailey, WC, Buist, AS, Conway, WA, Enright, PL,
Kanner, RE, O'hara, P & Owens, GR, 1994. Effects of smoking intervention and the use of an
Department
inhaled antic
This holinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study.
Freedom
JAMA, 272(19), pp. 1497-1505.
the
Anthonisen, NR, Manfreda, J, Warren, CP, Hershfield, ES, Harding, GK & Nelson, NA, 1987. Antibiotic
therapy in exacerb
by ations of chronic obstructive pulmonary disease, Ann Intern Med, 106(2),
pp. 196-204.
Anyanwu, AC, McGuire, A, Rogers, CA & Murday, AJ, 2001. Assessment of quality of life in lung
transplantation using a simple generic tool. Thorax, 56(3), pp. 218-22.
Anyanwu, AC, Rogers, CA & Murday J, 2000. Where are we today with pulmonary transplantation?
Current results from a national cohort. UK Cardiothoracic Transplant Audit Steering Group.
Transpl Int, 13 Suppl 1, pp. S245-6.
Asukai, Y, Baldwin, M & Mungapen, L, 2012. P84 utility values for COPD patients based on the EQ-5D
questionnaire from three indacaterol Phase II studies. Thorax, 67(Suppl 2), pp. A100-A101.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
193
Page 207 of 218
FOI 5155 - Document 4
Asukai, Y, Baldwin, M, Fonseca, T, Gray, A, Mungapen, L & Price, D, 2013. Improving clinical reality in
chronic obstructive pulmonary disease economic model ing. Pharmacoeconomics, 31(2), pp.
151-161.
Australia and New Zealand Cardiothoracic Organ Transplant Registry, 2016. 2016 Annual Report.
ANZCOTR, Sydney, viewed 8 Aug 2018, <http://www.anzcotr.org.au>.
Australian Institute of Health and Welfare, 2010, Asthma, chronic obstructive pulmonary disease and
other respiratory diseases in Australia (AIHW Cat. No. ACM 20.), Australian Institute of Health
and Welfare, Canberra, viewed 7 Aug 2018, <https://www.aihw.gov.au/reports/asthma-other-
chronic-respiratory-conditions/asthma-chronic-obstructive-pulmonary-disease-
and/contents/table-of-contents>.
Aventis Bering 2003, Alpha1-Proteinase Inhibitor (Human), IL, USA, viewed 17 July 2018,
Barker, AF, Iwata-Morgan, I, Oveson, L & Roussel, R, 1997. Pharmacokinetic study of alpha1
antitrypsin infusion in alpha<inf>1</inf>-antitrypsin deficiency, Chest, 112(3), pp
the . 607-613.
Barker, AF, Siemsen, F, Pasley, D, D'Silva, R & Buist, AS, 1994. Replacement therapy for hereditary
alpha1-antitrypsin deficiency. A program for long-term administration, Chest, 105(5), pp.
1406-1410.
under
(CTH) Care.
Barros-Tizon, JC, Torres, ML, Blanco, I & Martinez, MT, 2012. Reduction of severe exacerbations and
hospitalization-derived costs in alpha-1-antitrypsin-deficient patients treated with alpha-1-
antitrypsin augmentation therapy, Ther Adv Respir Dis, 6(2), pp. 67-78.
1982 Aged
Bergner, M, Hudson, LD & Conrad, DA, 1988. The cost and efficacy of home care for patients with
released
chronic lung disease. Med Care, 26:566-579.
Act and
Bernhard, N, Lepper, PM, Vogelmeier, C, Seibert, M, Wagenpfeil, S, Bals, R & Fahndrich, S, 2017.
Deterioration of quality of life is associated w
been ith the exacerbation frequency in individuals
with alpha-1-antitrypsin deficiency - analysis from the German Registry, Int J Chron Obstruct
Pulmon Dis, 12, pp. 1427-1437. has
Health
Bernspang, E, Diaz, S, Stoel, B, Wollmer, P, Sveger, T &
of Piitulainen, E, 2011. CT lung densitometry in
young adults with alpha-1-antitrypsin deficiency, Resp
Information ir Med, 105(1), pp. 74-79.
Blanco, I, Bueno, P, Diego, I, Perez-Ho
of landa, S, Casas-Maldonado, F, Esquinas, C & Miravitlles, M,
2017. Alpha-1 antitrypsin Pi*Z gene frequency and Pi*ZZ genotype numbers worldwide: an
update, Int J Chron Obstruct Pulmon Dis, 12, pp. 561-569.
document
Blanco, I, Bueno, P, Diego, I, Pérez-Holanda, S, Casas-Maldonado, F, Esquinas, C & Miravitlles, M,
2017. Alpha-1 antitrypsin Pi*Z gene frequen
Department cy and Pi*ZZ genotype numbers worldwide: an
This
update, International Journal
Freedom of Chronic Obstructive Pulmonary Disease, 12pp. 561-569.
the
Borg, S, Ericsson, A, Wedzicha, J, Gulsvik, A, Lundbäck, B, Donaldson, GC & Sul ivan, SD, 2004. A
computer simulation model of the natural history and economic impact of chronic obstructive
by
pulmonary disease. Value Health, 7(2):153–67.
Brantly, ML, Paul, LD, Miller, BH, Falk, RT, Wu, M & Crystal, RG, 1988. Clinical features and history of
the destructive lung disease associated with alpha-1-antitrypsin deficiency of adults with
pulmonary symptoms, Am Rev Respir Dis, 138(2), pp. 327-336.
Brode, SK, Ling, SC & Chapman, KR, 2012. Alpha-1 antitrypsin deficiency: a commonly overlooked
cause of lung disease, CMAJ : Canadian Medical Association Journal, 184(12), pp. 1365-1371.
Buist, AS, Vol mer, WM, Sul ivan, SD, Weiss, KB, Lee, TA, Menezes, AM, Crapo, RO, Jensen, RL &
Burney, PG, 2005. The burden of obstructive lung disease initiative (BOLD): rationale and
design. COPD, 2(2), pp.277-283.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
194
Page 208 of 218
FOI 5155 - Document 4
Busschbach, JJ, de Charro, FT, Horikx, PE, van den Bosch, JM & de la Rivière, AB, 1994. Measuring the
quality of life before and after bilateral lung transplantation in patients with cystic fibrosis.
Chest, 105(3), pp.911-917.
CADTH 2017, Alpha1-Proteinase Inhibitors for the Treatment of Alpha1Antitrypsin Deficiency: A
Review of Clinical Effectiveness, Cost Effectiveness, and Guidelines, The Canadian Agency for
Drugs and Technologies in Health, viewed 8 Aug 2018, <
https://www.cadth.ca/sites/default/files/pdf/htis/2017/RC0953%20Alpha1-
Proteinase%20Inhibitors%20Final.pdf>.
Calverley, PM, 2005. Minimal clinically important difference--exacerbations of COPD, Copd, 2(1), pp.
143-148.
Campos, M, Runken, M, Davis, A, Johnson, M & Buikema, A, 2015. Utilization and costs associated
with the prolastin direct alpha 1 proteinase inhibitor patient management program, Chest.
Conference: CHEST, 148(4), pp711A.
the
Campos, MA, Kueppers, F, Stocks, JM, Strange, C, Chen, J, Griffin, R, Wang-Smith, L & Brantly, ML,
2013. Safety and pharmacokinetics of 120 mg/kg versus 60 mg/kg weekly intravenous
infusions of alpha-1 proteinase inhibitor in alpha-1 antitrypsin deficiency: a multicenter,
under
randomized, double-blind, crossover study (SPARK), Copd, 10(6), pp. 687-695.
(CTH) Care.
Canadian Thoracic Society, 2012. Alpha-1 antitrypsin deficiency targeted testing and augmentation
therapy: A Canadian Thoracic Society clinical practice guideline. Can Respir J, 9(2), pp. 109-116.
1982 Aged
Carone, M, Bruletti, G, Bertella, E, Balestroni, G, Gatta N, Corda, L, Luisetti, M, & Balbi, B, 2011.
released
Quality of life evaluation in patients with alpha-1-antitripsin deficiency: A 3-year prospectic
Act
study. Europ Respir J. Conference: European Respiratory Society
and Annual Congress 2011.
Amsterdam Netherlands. Conference Publication: 38 (SUPPL. 55).
been
Chambers, DC, Yusen, RD, Cherikh WS, Goldfarb, SB, Kucheryavaya, AY, Khusch, K, Levvey, BJ, Lund,
LH, Meiser, B, Rossano, JW & Stehlik, J, 2017. The registry of the international society for heart
Health
and lung transplantation: thirty-fourth
has adult lung and heart-lung transplantation report—2017;
focus theme: allograft ischemic time, J Heart Lung
of Transplant, 36(10), pp.1047-1059.
Information
Chapman, K, Burdon, J, Pi tulainen, E, Sandhaus, R, Seersholm, N, Stocks, J, Stoel, B, Huang, L, Yao, Z,
Edelman, J & McElvaney, N, 201
of 5. Intravenous augmentation treatment and lung density in
severe ?1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial,
Lancet (london, england), 386(9991), pp. 360-368.
document
Chapman, KR, Bergeron, C, Bhutani, M, Bourbeau, J, Grossman, RF, Hernandez, P, McIvor, RA &
Mayers, I, 2013. Do we know the minimal c
Department linically important difference (MCID) for COPD
This
exacerbations?, Copd, 10(2), p
Freedom p. 243-249.
the
Chapman, KR, Stockley, RA, Dawkins, C, Wilkes, MM & Navickis RJ, 2009. Augmentation therapy for
alpha1 antitrypsin deficiency: a meta-analysis, COPD, 6(3), pp. 177-184.
by
Chorostowska-Wynimko, J, 2016. Disease Modification in Emphysema Related to Alpha-1 Antitrypsin
Deficiency, Copd, 13(6), pp. 807-815.
Clemens, S, Begum, N, Harper, C, Whitty, JA & Scuffham, PA, 2014. A comparison of EQ-5D-3L
population norms in Queensland, Australia, estimated using utility value sets from Australia,
the UK and USA, Qual Life Res, 23(8), pp.2375-2381.
Crapo, RO, Morris, AH & Gardner, RM, 1981. Reference spirometric values using techniques and
equipment that meet ATS recommendations. Am Rev Respir Disease, 123:659-64.
Crossley, D, Renton, M, Khan, M, Low, EV & Turner, AM, 2018. CT densitometry in emphysema: a
systematic review of its clinical utility, Int J Chron Obstruct Pulmon Dis, 13pp. 547-563.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
195
Page 209 of 218
FOI 5155 - Document 4
Crystal, RG, Brantly, ML, Hubbard, RC, Curiel, DT, States, DJ, & Holmes, MD, 1989. The alpha 1-
antitrypsin gene and its mutations. Clinical consequences and strategies for therapy, Chest,
95(1), pp. 196-208.
CSL Behring, 2017. Schedule 4 proposal supporting the addition of ZEMAIRA to the National Products
List, CSL Behring , Melbourne [Commercial in confidence].
Dawkins, P, Wood, A, Nightingale, P & Stockley, R, 2009. Mortality in alpha-1-antitrypsin deficiency
in the United Kingdom, Respir Med, 103(10), pp. 1540-1547.
Dawkins, PA, Dowson, LJ, Guest, PJ & Stockley, RA, 2003. Predictors of mortality in alpha1-
antitrypsin deficiency, Thorax, 58(12), pp. 1020-1026.
de Serres, F & Blanco, I, 2014. Role of alpha-1 antitrypsin in human health and disease, J Intern Med,
276(4), pp. 311-335.
de Serres, FJ, Blanco, I & Fernandez-Bustillo, E, 2003. Genetic epidemiology of alpha-1 antitrypsin
the
deficiency in North America and Australia/New Zealand: Australia, Canada, New Zealand and
the United States of America, Clin Genet, 64(5), pp. 382-397.
Deng, MC, De Meester, JM, Smits, JA, Heinecke, J, & Scheld HH, 2000. Effect of receiving a heart
under
transplant: analysis of a national cohort entered on to a waiting list, stratified by heart failure
(CTH) Care.
severity, BMJ,321:540-5.
Dirksen, A, Dijkman, JH, Madsen, F, Stoel, B, Hutchison, DC, Ulrik, CS, Skovgaard, LT, Kok-Jensen, A,
Rudolphus, A, Seersholm, N, Vrooman, HA, Reiber, JH, Hansen, NC, H
1982 eckscher,
Aged T, Viskum, K &
Stolk, J, 1999. A randomized clinical trial of alpha(1)-antitrypsin augmentation therapy, Am J
released
Respir Crit Care Med, 160(5 Pt 1), pp. 1468-1472.
Act and
Dirksen, A, Piitulainen, E, Parr, DG, Deng, C, Wencker, M, Shaker, SB & Stockley, RA, 2009. Exploring
the role of CT densitometry: a randomised
been study of augmentation therapy in alpha1-
antitrypsin deficiency, Eur Respir J, 33(6), pp. 1345-1353.
Health
Dolan, P, 1997. Modeling valuations for Euro
has Qol health states. Med Care, 35(11):1095–1108.
of
Donohue, JF, 2005. Minimal clinically important differences in COPD lung function, Copd, 2(1), pp.
Information
111-124.
of
Dowson, LJ, Guest, PJ, Hil , SL, Holder, RL & Stockley, RA, 2001. High-resolution computed
tomography scanning in alpha1-antitrypsin deficiency: relationship to lung function and health
status, Eur Respir J, 17(6), pp. 1
document 097-1104.
DYNAMO-HIA project: COPD prevalence, incidence and mortality 2000-2007. Data from the General
Department
Practice Research Database (GPRD) from the UK. Viewed 8 Aug 2018, <www.gprd.com>.
This Freedom
Ejiofor, SI & Stockley, RA, 2015. Health status measurements in alpha-1antitrypsin deficiency (AATD),
the
European Respiratory Journal. Conference: European Respiratory Society Annual Congress, 46.
by
Fletcher, MJ, Upton, J, Taylor-Fishwick, J, Buist, SA, Jenkins, C, Hutton, J, Barnes, N, Van Der Molen, T,
Walsh, JW, Jones, P & Walker, S, 2011. COPD uncovered: an international survey on the
impact of chronic obstructive pulmonary disease [COPD] on a working age population. BMC
Pub Health, 11(1), p.612.
Food and Drug Administration. 2003, Alpha1-Proteinase Inhibitor (Human): Zemaira, A. Behring,
Kankakee IL, viewed
<https://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedPro
ducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM172304.pdf>.
Fregonese, L & Stolk, J, 2008. Hereditary alpha-1-antitrypsin deficiency and its clinical consequences,
Orphanet J Rare Dis, 3pp. 16.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
196
Page 210 of 218
FOI 5155 - Document 4
Geertsma, A, TenVergert, E, Bonsel, GJ, de Boer, W & van der Bij, W, 1998. Does lung transplantation
prolong life? A comparison of survival with and without transplantation, J Heart Lung
Transplant, 17:511-516.
Gildea, TR, Shermock, KM, Singer, ME & Stol er, JK, 2003. Cost-effectiveness analysis of
augmentation therapy for severe α1-antitrypsin deficiency, Am J Respir Critic Care Med,
167(10), pp.1387-1392.
Global Initiative for Chronic Obstructive Lung Disease (GOLD),2017. GOLDCOPD, viewed 8 Aug 2018,
<http://goldcopd.org/>
Gotzsche, PC & Johansen, HK, 2016. Intravenous alpha-1 antitrypsin augmentation therapy for
treating patients with alpha-1 antitrypsin deficiency and lung disease, Cochrane Database Syst
Rev, 9pp. Cd007851.
Green, CE, Parr, DG, Edgar, RG, Stockley, RA & Turner, AM, 2016. Lung density associates with
survival in alpha 1 antitrypsin deficient patients, Respir Med, 112pp. 81-87.
the
Groen, H, Van der Bij, W, Koeter, GH & TenVergert, EM, 2004. Cost-Effectiveness of Lung
Transplantation in Relation to Type of End-Stage Pulmonary Disease. Am J Transplant, 4(7),
pp.1155-1162.
under
(CTH) Care.
Guo, B, Moga, C, Harstall, C & Schopflocher, D, 2016. A principal component analysis is conducted
for a case series quality appraisal checklist, J Clin Epidemiol, 69pp. 199-207.e192.
Guyot, P, Ades, AE, Ouwens, MJ & Welton, NJ, 2012. Enhanced secondary
1982 analysis o
Aged f survival data:
reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Method,
released
12(1), p.9.
Act and
Häggblom, J, Kettunen, K, Karjalainen, J, Heliövaara, M, Jousilahti, P & Saarelainen, S, 2015.
Prevalence of PI* Z and PI* S alleles of alpha
been -1-antitrypsin deficiency in Finland. Europ Clin
Resp J, 2(1), p.28829.
Health
Hatipoglu, U & Stoller, JK, 2016. alpha1-Anti
has trypsin Deficiency, Clin Chest Med, 37(3), pp. 487-504.
of
Hay, JW & Robin, ED, 1991. Cost-effectiveness of alpha-1 antitrypsin replacement therapy in
Information
treatment of congenital chronic obstructive pulmonary disease, Am J Public Health, 81(4), pp.
427-433.
of
Health Canada, 2017, Product Monograph: Prolsatin-C, G. Therapeutics, Mississauga OT, viewed
<http://grifols.com/document
document s/17006/186428/prolastinc-en.pdf/ba7f60ab-69ed-4af8-8351-
6f6924e33f06>.
Department
Hesselink, AE, van der, Windt, DAWM, Penninx, BWJH, Wijnhoven, HAH, Twisk, JWR, Bouter, LM &
This Freedom
can Eijk, JTM, 2006. What predicts change in pulmonary function and quality of life in asthma
the
or COPD? J Asthma, 43(7):513–519.
Hilleman, DE, Dewan, N,
by Malesker, M, Friedman, M, 2000. Pharmacoeconomic evaluation of COPD,
Chest, 118:1278–1285.
Holme, J & Stockley, RA, 2009. CT scan appearance, densitometry, and health status in protease
inhibitor SZ alpha1-antitrypsin deficiency, Chest, 136(5), pp. 1284-1290.
Hoogendoorn, M, 2011. Economic impact of COPD: empirical and model-based studies on the cost-
effectiveness of treatment options [dissertation].Erasmus University, Rotterdam.
Hoogendoorn, M, Feenstra, TL, Asukai, Y, Briggs, AH, Hansen, RN, Leidl, R, Risebrough, N, Samyshkin,
Y, Wacker, M & Rutten-van Mölken, MP, 2017. External validation of health economic decision
models for chronic obstructive pulmonary disease (COPD): report of the third COPD modeling
meeting, Value Health, 20(3), pp.397-403.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
197
Page 211 of 218
FOI 5155 - Document 4
Hoogendoorn, M, Rutten-van Mölken, MP, Hoogenveen, RT, Al, MJ & Feenstra, TL, 2011. Developing
and applying a stochastic dynamic population model for chronic obstructive pulmonary
disease, Value Health, 14(8), pp.1039-1047.
Hoogendoorn, M, Rutten-van Mölken, MPMH, Hoogenveen, RT, Van Genugten, MLL, Buist, AS,
Wouters, EFM & Feenstra, TL, 2005. A dynamic population model of disease progression in
COPD. Eur Respir J, 26(2), pp.223-233.
Horita, N, Miyazawa, N, Kojima, R, Inoue, M, Ishigatsubo, Y & Kaneko, T, 2015. Minimum clinically
important difference in diffusing capacity of the lungs for carbon monoxide among patients
with severe and very severe chronic obstructive pulmonary disease, Copd, 12(1), pp. 31-37.
Hosenpud, JD, Bennett, LE, Keck, BM, Boucek, MM, Novick, RJ, 2000. The Registry of the
International Society for Heart and Lung Transplantation: seventeenth official report—2000, J
Heart Lung Transplant, 19:909-31.
Hubbard, RC & Crystal, RG, 1988. Alpha-1-antitrypsin augmentation therapy for alph
the a-1-antitrypsin
deficiency, Am J Med, 84(6), pp. 52-62.
Jones, PW, 1994. Quality of life, symptoms and pulmonary function in asthma: long-term treatment
with nedocromil sodium examined in a controlled multicentre trial. N
under edocromil Sodium
Care.
Quality of Life Study Group, Eur Respir J, 7(1), pp. 55-62.
(CTH)
Jones, PW, 2005. St. George's Respiratory Questionnaire: MCID, COPD, 2(1):75–79.
Jones, PW, Brusselle, G, Dal Negro, RW, Ferrer, M, Kardos, P, Levy, ML, Pe
1982 rez, T, Cat
Aged aluna, JJS, van
der Molen, T, Adamek, L & Banik, N, 2011. Properties of the COPD assessment test in a cross-
released
sectional European study, Eur Respir J, 38:29–35.
Act and
Jones, PW, Quirk, FH, Baveystock, CM & Littlejohns, P, 1992. A self-complete measure of health
status for chronic airflow limitation. The St. Ge
been orge's Respiratory Questionnaire, Am Rev
Respir Dis, 145:1321–7.
Health
Jones, PW, Quirk, FH, Baveystock, CM, & L
has ittlejohns, P, 1992. A self-complete measure of health
status for chronic airflow limitation. Am Rev Respir
of Dis, 145: 1321–1327.
Information
Karl, FM, Hol e, R, Bals, R, Greulich, T, Jörres, RA, Karch, A, Koch, A, Karrasch, S, Leidl, R, Schulz, H &
Vogelmeier, C, 2017. Costs and h
of ealth-related quality of life in Alpha-1-Antitrypsin Deficient
COPD patients, Respir Res, 18(1), p.60.
Karl, FM, Hol e, R, Bals, R, Greulich, T
document , Jorres, RA, Karch, A, Koch, A, Karrasch, S, Leidl, R, Schulz, H,
Vogelmeier, C & Wacker, ME, 2017. Costs and health-related quality of life in Alpha-1-
Antitrypsin Deficient COPD patients, Respir Re
Department s, 18(1), pp. 60.
This Freedom
Kel y, E, Greene, CM, Carrol , TP, McElvaney, NG & O'Neil , SJ, 2010. Alpha-1 antitrypsin deficiency,
the
Respir Med, 104(6), pp. 763-772.
Kim, S-H, Oh, YM & Jo,
by M-W, 2014. Health-related quality of life in chronic obstructive pulmonary
disease patients in Korea, Health Qual Life Outcomes, 12.
Knebel, AR, Leidy, NK & Sherman, S, 1999. Health related quality of life and disease severity in
patients with alpha-1 antitrypsin deficiency, Qual Life Res, 8(4), pp. 385-391.
Köhnlein, T & Welte, T, 2008. Alpha-1 antitrypsin deficiency: pathogenesis, clinical presentation,
diagnosis, and treatment, Am J Med, 121(1), pp. 3-9.
Larsson C, 1978. Natural history and life expectancy in severe alpha 1-antitrypsin deficiency, PiZ.
Acta Med Scand, 204, pp.345-51.
Liberati, A, Altman, DG, Tetzlaff, J, Mulrow, C, Gotzsche, PC, Ioannidis, JP, Clarke, M, Devereaux, PJ,
Kleijnen, J & Moher, D, 2009. The PRISMA statement for reporting systematic reviews and
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
198
Page 212 of 218
FOI 5155 - Document 4
meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration,
BMJ, 339pp. b2700.
Lieberman, J, 2000. Augmentation therapy reduces frequency of lung infections in antitrypsin
deficiency: a new hypothesis with supporting data, Chest, 118(5), pp. 1480-1485.
Lomas, DA & Parfey, H, 2014. α1-Antitrypsin deficiency 4: molecular pathophysiology, Thorax, 59(6),
pp. 529-535.
Lundbäck B, Rönmark E, Jönsson E & Jonsson, AC, 1999. 10 year fol ow-up of the Obstructive Lung
Disease in Northern Sweden study’s (OLIN) first cohort. Am J Respir Crit Care Med, 159:134.
Manca, S, Rodriguez, E, Huerta, A, Torres, M, Lazaro, L, Curi, S, Pirina, P & Miravitlles, M, 2014.
Usefulness of the CAT, LCOPD, EQ-5D and COPDSS scales in understanding the impact of lung
disease in patients with alpha-1 antitrypsin deficiency, Copd, 11(5), pp. 480-488.
McElvaney, NG, Burdon, J, Holmes, M, Glanvil e, A, Wark, PA, Thompson, PJ, Hernandez, P, Chlumsky,
the
J, Teschler, H, Ficker, JH, Seersholm, N, Altraja, A, Makitaro, R, Chorostowska-Wynimko, J,
Sanak, M, Stoicescu, PI, Pi tulainen, E, Vit, O, Wencker, M, Tortorici, MA, Fries, M, Edelman,
JM & Chapman, KR, 2017. Long-term efficacy and safety of alpha1 proteinase inhibitor
treatment for emphysema caused by severe alpha1 antitrypsin defici
under ency: an open-label
Care.
extension trial (RAPID-OLE), Lancet Respir Med, 5(1), pp. 51-60.
(CTH)
McElvaney, NG, Stol er, JK, Buist, AS, Prakash, UB, Brantly, ML, Schluchter, MD & Crystal, RD, 1997.
Baseline characteristics of enrollees in the National Heart, Lung and Blood Institute Registry of
1982 Aged
alpha 1-antitrypsin deficiency. Alpha 1-Antitrypsin Deficiency Registry Study Group, Chest,
released
111(2), pp. 394-403.
Act and
McKenzie, D, Frith, PA, 2003. The COPDX Plan: Australian and New Zealand Guidelines for the
management of Chronic Obstructive Pulmonary Disease, Med J Aust, 178 Suppl, S7-39.
been
Miravitlles, M, Herr, C, Ferrarotti, I, Jardi, R, Rodriguez-Frias, F, Luisetti, M & Bals, R, 2010.
Laboratory testing of individuals with severe alpha1-antitryp
Health sin deficiency in three European
has
centres, Eur Respir J, 35(5), pp. 960-968.
of
Moayeri F, Hsueh YS, Clarke P, Hua X & Dunt D, 2016
Information . Health State Utility Value in Chronic
Obstructive Pulmonary Disease (COPD); The Chal enge of Heterogeneity: A Systematic Review
of
and Meta-Analysis, COPD, 13(3):380-98.
Mullins, CD, Huang, X, Merchant, S, Stoller, JK & Alpha One Foundation Research Network Registry
document
Investigators, 2001. The direct medical costs of α1-antitrypsin deficiency, Chest, 119(3),
pp.745-752.
This
Department
Mullins, CD, Wang, J & Stoller, JK,
Freedom 2003. Major components of the direct medical costs of alpha1-
antitrypsin deficiency, Chest, 124(3), pp. 826-831.
the
National Institute for Health and Clinical Excellence, 2008. Guide to the methods of technology
by
appraisal. National Institute for Health and Clinical Excellence, London.
National Institute for Health and Clinical Excellence, 2010. Chronic obstructive pulmonary disease:
management of chronic obstructive pulmonary disease in adults in primary and secondary
care (partial update). Clinical Guideline CG101, NICE, London, viewed 7 Aug 2018, <
https://www.nice.org.uk/guidance/cg101>.
National Institute for Health and Clinical Excellence, 2011. Costing report: chronic obstructive
pulmonary disease. Clinical Guideline CG101, NICE, London, viewed 7 Aug 2018.
National Institute for Health Research, 2014, Alpha-1 antitrypsin (Respreeza) for emphysema
associated with alpha-1 antitrypsin deficiency-maintenance therapy, NIHR Horizon Scanning
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
199
Page 213 of 218
FOI 5155 - Document 4
Centre, Birmingham, viewed 7 Aug, < http://www.io.nihr.ac.uk/wp-
content/uploads/migrated/2709.a214236d.Alpha1antitrypsin_Nov14.pdf>.
NIHR Horizon Scanning Centre, 2014. Briefing note: Alpha-1 antitrypsin (Respreeza) for emphysema
associated with alpha-1 antitrypsin deficiency – maintenance therapy.
Norman, R, Church, J, van den Berg, B & Goodall, S, 2013. Australian health-related quality of life
population norms derived from the SF-6D, Aust N Z J Public Health, 37(1), pp.17-23.
Oostenbrink, JB & Rutten-van Molken, MP, 2004. Resource use and risk factors in high-cost
exacerbations of COPD, Respir Med, 98:883–91.
Oostenbrink, JB, Rutten-van Mölken, MP, Monz, BU & FitzGerald, JM, 2005. Probabilistic Markov
model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different
countries, Value health, 8(1), pp.32-46.
Paraskeva, MA, Levin, KC, Westall, GP & Snell, GI, 2018. Lung transplantation in Australia, 1986-2018:
the
more than 30 years in the making, MJA, 208(10), pp.445-450.
Parr, DG, Stoel, BC, Stolk, J & Stockley, RA, 2006. Validation of computed tomographic lung
densitometry for monitoring emphysema in alpha1-antitrypsin deficiency, Thorax, 61(6), pp.
under
485-490.
(CTH) Care.
Pharmacy and Therapeutics, 2010. Alpha(1)-Proteinase Inhibitor (Human), Pharmacy and
Therapeutics, 35(3 Section 2), pp. 2-6.
1982 Aged
Pickard, AS, Yang, Y & Lee, TA, 2011. Comparison of health-related quality of life measures in chronic
obstructive pulmonary disease, Health Qual Life Outcomes, 9(1
released ), pp.26.
Act and
Piitulainen, E, Bernspang, E, Bjorkman, S & Berntorp, E, 2003. Tailored pharmacokinetic dosing
allows self-administration and reduces the cost of IV augmentation therapy with human
been
alpha(1)-antitrypsin, Eur J Clin Pharmacol, 59(2), pp. 151-156.
Price, D, Gray, A, Gale, R, Asukai, Y, Mungapen, L, Lloyd, A, Peters, L, Neidhardt, K & Gantner, T, 2011.
has
Health
Cost-utility analysis of indacaterol in Germany: a once-daily maintenance bronchodilator for
of
patients with COPD, Respir Med, 105(11), pp.1635-1647.
Information
Punekar, YS, Rodriguez-Roisin, R, Sculpher, M, Jones, P & Spencer, M, 2007. Implications of chronic
of
obstructive pulmonary disease (COPD) on patients’ health status: a western view, Respir Med,
101(3):661–669.
document
Quanjer, PH, Tammeling, GJ, Cotes, JE, Pedersen, OF, Peslin, R & Yernault, JC, 1993. Lung volumes
and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests,
Department
European Community for Steel and Coal. Official Statement of the European Respiratory
This Freedom
Society, Eur Respir J Suppl, 16pp. 5-40.
the
Rahaghi, FF, Sandhaus, RA, Brantly, ML, Rouhani, F, Campos, MA, Strange, C, Hogarth, DK, Eden, E,
Stocks, JM, Krowk
by a, MJ & Stoller, JK, 2012. The prevalence of alpha-1 antitrypsin deficiency
among patients found to have airflow obstruction, COPD, 9(4), pp. 352-358.
Ramsey, SD, Patrick, DL, Albert, RK, Larson, EB, Wood, DE & Raghu G, 1995. The cost-effectiveness of
lung transplantation: a pilot study. University of Washington Medical Center Lung Transplant
Study Group, Chest, 108(6), pp.1594-601.
Ranes, J & Stoller, JK, 2005. A review of alpha-1 antitrypsin deficiency, Semin Respir Crit Care Med,
26(2), pp. 154-166.
Rutten-van Molken, M & Lee, TA, 2006. Economic modeling in chronic obstructive pulmonary
disease, Proc Am Thorac Soc, 3:630–634.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
200
Page 214 of 218
FOI 5155 - Document 4
Rutten-van Mölken, MP, Hoogendoorn, M & Lamers, LM, 2009. Holistic preferences for 1-year
health profiles describing fluctuations in health, Pharmacoeconomics, 27(6), pp.465-477.
Rutten-van Molken, MP, Oostenbrink, JB, Miravitlles, M & Monz, BU, 2007. Modelling the 5-year
cost effectiveness of tiotropium, salmeterol and ipratropium for the treatment of chronic
obstructive pulmonary disease in Spain, Eur J Health Econ, 8(2), pp.123e35.
Rutten-Van Molken, MPMH, Oostenbrink, JB, Tashkin, DP, Burkhart, D & Monz, BU, 2006. Does
quality of life of COPD patients as measured by the generic EuroQol five-dimension
questionnaire differentiate between COPD severity stages?, Chest, 130(4):1117–1128.
Samyshkin, Y, Kotchie, RW, Mörk, AC, Briggs, AH & Bateman, ED, 2014. Cost-effectiveness of
roflumilast as an add-on treatment to long-acting bronchodilators in the treatment of COPD
associated with chronic bronchitis in the United Kingdom, Eur J Health Econ, 15(1), pp.69-82.
Sandhaus, RA, Stocks, J, Rouhani, FN, Brantly, M & Strauss, P, 2014. Biochemical efficacy and safety
of a new, ready-to-use, liquid alpha-1-proteinase inhibitor, GLASSIA (alph
the a1-proteinase
inhibitor (human), intravenous), Copd, 11(1), pp. 17-25.
Sandhaus, RA, Turino, G, Brantly, ML, Campos, M, Cross, CE, Goodman, K, Hogarth, DK, Knight, SL,
Stocks, JM, Stol er, JK, Strange, C & Teckman, J, 2016. The Diagnosis a
under nd Management of
Care.
Alpha-1 Antitrypsin Deficiency in the Adult, COPD, 3(3), pp. 668-682. (CTH)
Schluchter, MD, Stoller, JK, Barker, AF, Buist, AS, Crystal, RG, Donohue, JF, Fallat, RJ, Turino, GM,
Vreim, CE & Wu, MC, 2000. Feasibility of a clinical trial of augmentation therapy for alpha(1)-
1982 Aged
antitrypsin deficiency. The Alpha 1-Antitrypsin Deficiency Registry Study Group, Am J Respir
released
Crit Care Med, 161(3 Pt 1), pp. 796-801.
Act and
Schmidt, EW, Rasche, B, Ulmer, WT, Konietzko, N, Becker, M, Fal ise, JP, Lorenz, J & Ferlinz, R, 1988.
Replacement therapy for alpha-1-protease inhibitor deficiency in PiZ subjects with chronic
been
obstructive lung disease, Am J Med, 84(6), pp. 63-69.
Schwaiblmair, M, Vogelmeier, C & Fruhmann, G, 1997. Long-term a
Health ugmentation therapy in twenty
has
patients with severe alpha-1-antitrypsin deficiency--three-year follow-up, Respiration, 64(1),
of
pp. 10-15.
Information
Sclar, DA, Evans, MA, Robison, LM, Skaer, TL, 2012. Alpha1-Proteinase inhibitor (human) in the
of
treatment of hereditary emphysema secondary to alpha1-antitrypsin deficiency: number and
costs of years of life gained, Clin Drug Investig, 32:353–360.
document
Seersholm, N, Kok-Jensen, A & Dirksen, A, Survival of patients with severe alpha 1-antitrypsin
deficiency with special reference to non-index cases.[Erratum appears in Thorax 1994
Department
Nov;49(11):1184], [Erratum appears in Thorax 1998 Jan;53(1):78], Thorax, 49(7), pp. 695-698.
This Freedom
Seersholm, N, Wencker, M, Banik, N, Viskum, K, Dirksen, A, Kok-Jensen, A & Konietzko, N, 1997.
the
Does alpha1-antitrypsin augmentation therapy slow the annual decline in FEV1 in patients
with severe hered
by itary alpha1-antitrypsin deficiency? Wissenschaftliche Arbeitsgemeinschaft
zur Therapie von Lungenerkrankungen (WATL) alpha1-AT study group, Eur Respir J, 10(10), pp.
2260-2263.
Sharples, LD, Taylor, GJ, Karnon, J, Caine, N, Buxton, M, McNeil, K, Wallwork, J, 2001. A model for
analyzing the cost of the main clinical events after lung transplantation, J Heart Lung
Transplant, 20(4), pp.474-82.
Shermock, KM, Gildea, TR, Singer, M, Stoller, JK, 2005. Cost-effectiveness of population screening for
alpha-1 antitrypsin deficiency: a decision analysis, COPD, 2(4), pp.411–418.
Sin, DD, Golmohammadi, K, Jacobs, P, 2004. Cost-effectiveness of inhaled corticosteroids for chronic
obstructive pulmonary disease according to disease severity, Am J Med, 116, pp.325–331.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
201
Page 215 of 218
FOI 5155 - Document 4
Singer, LG, Chowdhury, N, Chaparro, C & Hutcheon, MA, 2009. Post-lung transplant health-related
quality of life: Perception and reality, Journal of Heart and Lung Transplantation. Conference:
29th Annual Meeting and Scientific Sessions of the International Society for Heart and Lung
Transplantation. Conference Publication, Paris, 28 (2 SUPPL. 1), pp. S127.
Singer, LG, Chowdhury, NA, Faughnan, ME, Granton, J, Keshavjee, S, Marras, TK, Tullis, DE, Waddell,
TK & Tomlinson, G, 2015. Effects of recipient age and diagnosis on health-related quality-of-
life benefit of lung transplantation, Am J Respir Crit Care Med, 192(8), pp.965-973.
Singh, SJ, Jones, PW, Evans, R & Morgan, MD, 2008. Minimum clinically important improvement for
the incremental shuttle walking test, Thorax, 63(9), pp. 775-777.
Singh, SJ, Morgan, MD, Scott, S, Walters, D & Hardman, AE, 1992. Development of a shuttle walking
test of disability in patients with chronic airways obstruction, Thorax, 47(12), pp. 1019-1024.
Spencer, M, Briggs, AH, Grossman, RF & Rance L, 2005. Development of an economic model to
assess the cost effectiveness of treatment interventions for chronic obstruct
the ive pulmonary
disease, Pharmacoeconomics, 23, pp.619–637.
Stahl, E, Jansson, S-A, Jonsson, A-C, Svensson, K, Lundback, B & Andersson, F, 2003. Health-related
quality of life, utility, and productivity outcomes instruments: ease of co
under mpletion by subjects
Care.
with COPD, Health Qual Life Outcomes, 1, pp.18.
(CTH)
Ståhl, E, Lindberg, A, Rönmark, E, Svensson, K, Jansson, SA, Andersson, F & Lundbäck, B, 2001. The
level of health-related quality of life in patients with COPD and its dependence on age, gender
1982 Aged
and disease severity, Eur Respir J, 18(Suppl 33), pp.S184.
released
Starkie, HJ, Briggs, AH, Chambers, MG & Jones, P, 2011. Predicting EQ-5D values using the SGRQ,
Act and
Value Health, 14(2), pp.354–360.
Sterne, JA, Hernán, MA, Reeves, BC, Savović, J, Berkm
been an, ND, Viswanathan, M, Henry, D, Altman, DG,
Ansari, MT, Boutron, I, Carpenter, JR, Chan, A-W, Churchil , R, Deeks, JJ, Hróbjartsson, A,
Kirkham, J, Jüni, P, Loke, YK, Pigott, TD, Ramsay, CR, Regid
Health or, D, Rothstein, HR, Sandhu, L,
has
Santaguida, PL, Schünemann, HJ, Shea, B, Shrier, I, Tugwel , P, Turner, L, Valentine, JC,
of
Waddington, H, Waters, E, Wel s, GA, Whiting, PF & Higgins, JP, 2016a. ROBINS-I: a tool for
Information
assessing risk of bias in non-randomised studies of interventions, BMJ, 355pp.
of
Steuten, L, Vrijhoef, B, Merode, FV, Wesseling, GJ & Spreeuwenberg, C, 2006. Evaluation of a
regional disease management programme for patients with asthma or chronic obstructive
pulmonary disease, Int J Qual H
document ealth Care, 18(6), pp.429-436.
Stockley, RA, 2015. Antitrypsin Deficiency Assessment and Programme for Treatment (ADAPT): The
Department
United Kingdom Registry, COPD, 12(Suppl 1), pp.63-68.
This Freedom
Stocks, J, Brantly, M, Wang-Smith, L, Campos, M, Chapman, K, Kueppers, F, Sandhaus, R, Strange, C
the
& Turino, G, 2010a. Pharmacokinetic comparability of Prolastin®-C to Prolastin® in alpha?-
antitrypsin deficie
by ncy: a randomized study, BMC clinical pharmacology, 10pp. 13.
Stocks, JM, Brantly, M, Pol ock, D, Barker, A, Kueppers, F, Strange, C, Donohue, JF & Sandhaus, R,
2006. Multi-center study: the biochemical efficacy, safety and tolerability of a new alpha1-
proteinase inhibitor, Zemaira, Copd, 3(1), pp. 17-23.
Stocks, JM, Brantly, ML, Wang-Smith, L, Campos, MA, Chapman, KR, Kueppers, F, Sandhaus, RA,
Strange, C & Turino, G, 2010b. Pharmacokinetic comparability of Prolastin(R)-C to Prolastin(R)
in alpha(1)-antitrypsin deficiency: a randomized study, BMC Clin Pharmacol, 10pp. 13.
Stolk, J, Ng, WH, Bakker, ME, Reiber, JH, Rabe, KF, Putter, H & Stoel, BC, 2003. Correlation between
annual change in health status and computer tomography derived lung density in subjects
with alpha1-antitrypsin deficiency, Thorax, 58(12), pp. 1027-1030.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
202
Page 216 of 218
FOI 5155 - Document 4
Stol er, JK, Fal at, R, Schluchter, MD, O'Brien, RG, Connor, JT, Gross, N, O'Neil, K, Sandhaus, R &
Crystal, RG, 2003. Augmentation therapy with alpha1-antitrypsin: patterns of use and adverse
events, Chest, 123(5), pp. 1425-1434.
Stoller, JK, Smith, P, Yang, P & Spray, J, 1994. Physical and social impact of alpha 1-antitrypsin
deficiency: results of a survey, Cleve Clin J Med, 61(6), pp. 461-467.
Strauss, MJ, Conrad, D, LoGerfo, JP, Hudson, LD & Bergner M, 1986. Cost and outcome for patients
with chronic obstructive lung disease, Med Care, 24, pp.915-924.
Tanash, HA, Nilsson, PM, Nilsson, JA & Piitulainen, E, 2010. Survival in severe alpha-1-antitrypsin
deficiency (PiZZ), Respir Res, 11pp. 44.
Tanash, HA, Riise, GC, Ekstrom, MP, Hansson, L & Piitulainen, E, 2014. Survival benefit of lung
transplantation for chronic obstructive pulmonary disease in Sweden, Annals of Thoracic
Surgery, 98(6), pp.1930-1935.
the
Tanash, HA, Riise, GC, Hansson, L, Nilsson, PM & Piitulainen, E, 2011. Survival benefit of lung
transplantation in individuals with severe alpha1-anti-trypsin deficiency (PiZZ) and
emphysema, J Heart Lung Transplant, 30(12), pp. 1342-1347. under
TenVergert, EM, Essink-Bot, ML, Geertsma, A, van Enckevort, PJ, de Boer, WJ, van der, BW, 1998.
(CTH) Care.
The effect of lung transplantation on health-related quality of life: a longitudinal study, Chest,
113, pp.358–364.
The Alpha-1-Antitrypsin Deficiency Registry Study Group, 1998. Surviv
1982 al and F
Aged EV1 decline in
individuals with severe deficiency of alpha1-antitrypsin. The Alpha-1-Antitrypsin Deficiency
released
Registry Study Group, Am J Respir Crit Care Med, 158(1), pp. 49-59.
Act and
Therapeutic Goods Administration 2016, PROLASTIN®-C Alpha1-Proteinase Inhibitor (Human)
Registered Product Information Therapeutic G
been oods Administration, viewed 10 August 2018,
<https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2016-PI-
02743-1&d=201808101016933>.
has
Health
Thomas, M, Radwan, A, Stonham, C & Marshall, S
of , 2014. COPD exacerbation frequency,
pharmacotherapy and resource use: an observational
Information study in UK primary care, COPD, 11(3),
pp.300-309.
of
Toel e, BG, Xuan, W, Bird, TE, Abramson, MJ, Atkinson, DN, Burton, DL, James, AL, Jenkins, CR, Johns,
DP, Maguire, GP & Musk, AW, 2013. Respiratory symptoms and illness in older Australians: the
document
Burden of Obstructive Lung Disease (BOLD) study. Med J Aust, 198(3), pp.144-148.
Tonelli, AR & Brantly, ML, 2010. Augmentation the
Department rapy in alpha-1 antitrypsin deficiency: advances
This
and controversies, Ther Adv R
Freedom espir Dis, 4(5), pp. 289-312.
the
Tonelli, AR, Rouhani, F, Li, N, Schreck, P & Brantly, ML, 2009. Alpha-1-antitrypsin augmentation
therapy in deficient individuals enrolled in the Alpha-1 Foundation DNA and Tissue Bank, Int J
by
Chron Obstruct Pulmon Dis, 4pp. 443-452.
Torrance, GW, 1976. Social preferences for health states: an empirical evaluation of three
measurement techniques, Socio-Econ Planning Sci, 10(3), pp.129–136.
Tsuchiya, A, Ikeda, S, Ikegami, N, Nishimura, S, Sakai, I, Fukuda, T, Hamashima, C, Hisashige, A &
Tamura, M, 2002. Estimating an EQ-5D population value set: the case of Japan, Health Econ,
11(4), pp.341-353.
UK Department of Health, 2011. NHS reference costs 2009–2010. Viewed 8 Aug 2018, <
https://www.gov.uk/government/publications/nhs-reference-costs-2009-2010>.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
203
Page 217 of 218
FOI 5155 - Document 4
van den Berg, JW, Geertsma, A, van der Bij, WIM, KoËter, GH, de Boer, WJ, Postma, DS & ten Vergert,
EM, 2000. Bronchiolitis obliterans syndrome after lung transplantation and health-related
quality of life, Am J Respir Crit Care Med,161(6), pp.1937–1941.
Van Enckevort, PJ, TenVergert, EM, Bonsel, GJ, Geertsma, A, Van Der Bij, W, De Boer, WJ,
Koopmanschap, MA, Al, MJ & Ruttten, FF, 1998. Technology assessment of the Dutch lung
transplantation program, Int J Technol Asses Health Care, 14(2), pp.344–356.
Vasiliadis, HM, Collet, JP, Penrod, JR, Ferraro & P, Poirier, C, 2005. A cost-effectiveness and cost-
utility study of lung transplantation, J Heart Lung Transplant, 24(9), pp.1275–1283.
Vijayasaratha, K & Stockley, RA, 2012. Relationship between frequency, length, and treatment
outcome of exacerbations to baseline lung function and lung density in alpha-1 antitrypsin-
deficient COPD, Int J Chron Obstruct Pulmon Dis, 7pp. 789-796.
Von Neumann, J & Morgenstern, O, 1944. Theory of games and economic behaviour. Princeton
University Press, Princeton.
the
Walters, SJ & Brazier, JE, 2005. Comparison of the minimally important difference for two health
state utility measures: EQ-5D and SF-6D, Qual Life Res, 14(6), pp.1523–1532.
under
Wencker, M, Banik, N, Buhl, R, Seidel, R & Konietzko, N, 1998. Long-term treatment of alpha1-
(CTH) Care.
antitrypsin deficiency-related pulmonary emphysema with human alpha1-antitrypsin.
Wissenschaftliche Arbeitsgemeinschaft zur Therapie von Lungenerkrankungen (WATL)-alpha1-
AT-study group, European Respiratory Journal, 11(2), pp. 428-433.
1982 Aged
Wewers, MD, Casolaro, MA, Sellers, SE, Swayze, SC, McPhaul, KM, Wittes, JT & Crystal, RG, 1987.
released
Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema, N Engl J
Act and
Med, 316(17), pp. 1055-1062.
Wu, M, Zhao, Q, Chen, Y, Fu, C & Xu, B, 2015. Qualit
been y of life and its association with direct medical
costs for COPD in urban China, Health Qual Life Outcomes, 13(1), pp.57.
Health
Yang, IA, Brown, JL, George, J, Jenkins, S, M
has cDonald, CF, McDonald, VM, Phillips, K, Smith, BJ, Zwar,
NA & Dabscheck, E, 2017. COPD-X Australian an
of d New Zealand guidelines for the diagnosis
and management of chronic obstructive pulmonary d
Information isease: 2017 update, Med J Aust, 207(10),
pp. 436-442.
of
Zacherle, E, Noone, JM, Runken, MC & Blanchette, CM, 2015. Health care cost and utilization
associated with alpha-1 antitrypsin deficiency among a cohort of medicare beneficiaries with
document
COPD, Value Health, 18, pp.A664.
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
204
Page 218 of 218

FOI 5155 - Document 5
Public Summary Document
Application No. 1530 – Purified human alpha1-proteinase inhibitor
for the treatment of alpha1-proteinase inhibitor deficiency, leading
to chronic obstructive pulmonary disease
the
Applicant:
National Blood Authority (NBA)
Date of MSAC consideration: MSAC 74th Meeting, 23-24 November 2018
under
Context for decision: MSAC makes its advice in accordance with its Terms of Reference,
Care.
(CTH)
visit the MSAC website
1.
Purpose of application
Aged
released 1982
An application requesting National Product List (NPL) blood product listing of purified
Act and
human alpha1-proteinase inhibitor (A1-PI) for the treatment of A1-PI deficiency, leading to
chronic obstructive pulmonary disease (COPD), was received from the National Blood
been
Authority (NBA) by the Department of Health.
Health
2.
MSAC’s advice to the Minist
has
er of
After considering the strength of the available evidence
Information in relation to comparative safety,
clinical effectiveness and cost-effectiveness, MSAC did not support A1-PI for the treatment
of
of A1-PI deficiency. MSAC recognised the large unmet clinical need and the evidence of a
radiologically detectable treatment effect, but was concerned with the weak evidentiary basis
provided to suggest that changes in C
document T density predicts clinically meaningful health
outcomes. MSAC also advised that, even with favourable assumptions regarding estimates of
possible health outcomes of A1-PI treatment, the
Department economic evaluation generated
This Freedom
unacceptably large incremental cost-effectiveness ratios at the prices proposed by the
sponsors.
the
by
3.
Summary of consideration and rationale for MSAC’s advice
MSAC noted the impact that severe A1-PI deficiency (serum A1≤11μM) with emphysema
(FEV1<80%) has on patients and their carers, resulting in strong consumer support for the
proposed treatment both in Australia and overseas.
The proposed treatment is lifelong intravenous blood augmentation therapy via weekly
infusions of purified human A1-PI for ex- or never-smoking patients. MSAC noted that the
two alternative products are considered to be essentially bioequivalent. MSAC noted that the
recommended dosing is 60mg/kg per week, but that there are ongoing clinical trials
investigating optimal dosing regimens, with dosing up to 120mg/kg per week. MSAC noted
1
Page 1 of 18
FOI 5155 - Document 5
that if the required dose is higher, then the overall cost would increase if the current price per
mg is maintained.
MSAC noted that augmentation therapy with A1-PI is not currently funded or reimbursed in
private or public settings in Australia for this or any other clinical indication.
MSAC noted the estimated prevalence of carriers of alleles related to A1-PI deficiency in the
Australian population is 1 in 8.9 individuals. The PiZZ allele (with a prevalence of 1 in
5584), contributes to the greatest burden; however, not all people with PiZZ A1-PI deficiency
will go on to develop severe emphysema. MSAC noted that the estimated number of people
meeting the criteria for treatment with A1-PI in Australia in 2018 was s47(1) Treatment is
lifelong and not curative; therefore, the number of patients being treated i
(b) s expected to
moderately cumulative increase over time.
MSAC noted that the comparator intervention for patients with severe A1-PIdeficiency and
emphysema is best supportive care (BSC).
the
MSAC noted that, overall, it appears that A1-PI is safe, with most adverse events being
related to the underlying disease.
under
(CTH) Care.
MSAC noted that there are no statistically significant differences between A1-PI and placebo
in relation to mortality, exacerbation of COPD, hospitalisation due to COPD exacerbation,
quality of life (St. George's Respiratory Questionnaire), respiratory function (FEV1), exercise
1982 Aged
capacity (incremental shuttle walk test) or carbon monoxide diffusion capacity (DLCO).
released
Act
MSAC noted that the only statistically significant difference observed in
and clinical trials was
for CT-measured lung density, which favoured A1-PI therapy compared with placebo.
MSAC noted that recommending public funding of
been A1-PI products requires accepting that
effects on CT-measured lung density have been demonstrated to be a surrogate for effects on
outcomes known to be clinically meaningf
has ul, including respirat
Health ory function, quality of life,
overall survival, or quality-adjusted life-years (QAL
of Ys). However, even the clinical
significance of the observed difference in CT-measured l
Information ung density is uncertain, as minimal
clinically important differences (MCIDs) for changes in this surrogate have not been
of
established in the peer-reviewed literature.
MSAC noted the claim that A1-PI
document therapy meets three of the four criteria warranting Rule of
Rescue. However, it is unclear whether CT-measured lung density is a sufficiently
informative surrogate for the Rule of Rescue crit
Department erion of ‘worthwhile clinical improvement’.
This Freedom
MSAC noted that CT lung density calculations are not routinely performed in Australia,
the
although it is likely all modern scanners could be equipped to do so with access to necessary
software (noting that the
by cost of software is unknown).
A1-PI is known to be ineffective in smokers. Strict requirements would therefore be needed
to ensure use is limited to non-smokers (of tobacco and/or cannabis).
MSAC noted that the treatment cost with A1-PI is high (approximately s47(1)(b) per patient
per year) for the patient’s lifetime and the base case modelled incremental cost-effectiveness
ratio (ICER) is s47(1)(b) per QALY gained using a weighted average price for the two
available A1-PI therapies. MSAC advised that this ICER/QALY was unacceptably large and
based on assumptions of long-term clinical effect that favoured the intervention, and
substantial price reductions would be required to bring it within an acceptable range.
2
Page 2 of 18
FOI 5155 - Document 5
MSAC noted that the assessment group attempted to improve the modelled cost-effectiveness
of the A1-PI products by applying an evidence-based stopping rule for patients who
demonstrate limited treatment response to A1-PI therapy. In the model, 113/1,000 individuals
in the cohort progress from no decline or slow decline to rapid decline, despite being on A1-
PI therapy for four years – the A1-PI therapy costs for these individuals beyond four years
was then removed from the model. However, this was only associated with a modest
improvement in cost-effectiveness and the ICER remained unacceptably large
(s47(1)(b) /QALY compared with s47(1)(b) /QALY for the base case).
MSAC also noted that an additional univariate sensitivity analysis (performed by the
assessment group by changing specific transitions from FEV1 >50 to FEV1<50 to remove a
modelled treatment effect on FEV1 which contradicted the results of the randomised trials)
did not have a major impact on the ICER. If both A1-PI therapy and BSC arms had FEV1
annual probability declines of s47(1) then the ICER would increase from s47(1)(b) /QALY to
s47(1)(b) /QALY.
(b)
the
MSAC noted that there is significant uncertainty regarding the number of patients who will
be diagnosed with A1-PI deficiency if the A1-PI products are available on the NPL. The
NBA would need to be able to negotiate an overall risk sharing arrangeme
under nt with suppliers to
mitigate this financial risk.
(CTH) Care.
MSAC concluded that there is a clear physiological effect on lung density which is detectable
radiologically; however, there is no basis on which to draw a large clini
1982 cal effec
Aged t, and thus no
evidence of patient-relevant outcomes.
released
Act and
MSAC again acknowledged the high priority the public consultation feedback gave to
meeting the clinical need that the applicant claims will be helped by this intervention, but
been
considered that the evidence was inadequate to justify the therapeutic claims made in the
application.
has
Health
4.
Background
Information
of
Augmentation therapy with any A1-PI therapy is not currently funded or reimbursed in
of
private or public settings in Australia (for this or any other clinical indication).
5.
Prerequisites to implem
document
entation of any funding advice
PROLASTIN-C and Zemaira (marketed as Resp
Department reeza in Europe), are two augmentation
therapy products r
This egistered on the
Freedom Australian Register of Therapeutic Goods (ARTG) in
Australia. The two therapies consist of the same components with slightly different eligibility
the
criteria (Table 1).
by
Table 1 Approved augmentation therapies and their indications
Product
ARTG ID and details
PROLASTIN-C
ARTG ID 234553: indicated to increase serum A1-PI levels in adults with congenital deficiency of alpha-
1 anti-trypsin and with clinically significant emphysema (FEV1 less than 80%). The data for clinical
ef icacy of PROLASTIN-C is derived from changes in the biomarkers alpha-1 anti-protease level and CT
lung density. Ef icacy on FEV1 or patient relevant endpoints such as quality of life or pulmonary
exacerbations has not been established in randomised clinical trials. Clinical trials have only included
patients who were not smoking.
Zemaira
ARTG ID 273182: indicated for maintenance treatment, to slow the progression of emphysema in adults
with documented severe A1-PI deficiency (A1-PI less than 11 μM) and progressive lung disease.
Patients are to be under optimal pharmacologic and non-pharmacologic treatment.
Abbreviations:
ARTG = Australian Register of Therapeutic Goods,
FEV1 = forced expiratory volume in 1 second,
μM = micromolar.
3
Page 3 of 18
FOI 5155 - Document 5
6.
Proposal for public funding
Augmentation therapy with A1-PI is proposed for reimbursement on the NPL, managed by
the NBA. As such, no Medicare Benefits Schedule item descriptor is required.
7.
Summary of public consultation feedback/consumer issues
Six associations provided targeted feedback, and one individual provided non-targeted
feedback on this consultation. All respondents using the feedback form ‘strongly agreed’ with
the clinical claim made by the applicant and argued the urgent priority to address the unmet
clinical need.
8.
Proposed intervention’s place in clinical management
The population to be considered in this assessment is ex- or never-smoking patients with
emphysema (defined as FEV
the
1 <80%) and severe A1-PI deficiency (defined as serum A1
levels ≤11 μM (approximately 59 mg/dL); Hatipoglu and Stoller 2016).
Patients with A1-PI deficiency are currently managed with best supportive care (BSC). BSC
under
includes pharmacological strategies (e.g. inhaled medications) and non-pharmacological
Care.
(CTH)
strategies (e.g. pulmonary rehabilitation and physical activity) aimed at providing
symptomatic relief. The current (Figure 1) and proposed (Figure 2) clinical management
algorithms are presented below.
1982 Aged
released
Act and
Patients with
emphysema and FEV1
<80%
been
has
Health
Information
of
of Investigated for A1-
PI deficiency with
document
serum levels and
genotyping
This Freedom
Department
the
by
Optimal
Optimal
pharmacological
Documented A1-PI
pharmacological
therapy and best
Yes
deficiency
No
therapy and best
supportive care
supportive care
Figure 1 Current clinical management algorithm for patients with emphysema and FEV1 <80%
4
Page 4 of 18
FOI 5155 - Document 5
Patients with
emphysema and FEV1 <
80%
Investigated for A1-PI
deficiency with serum
levels and genotyping
Optimal
pharmacological
Currently
yes
therapy and
the
smoking
assistance with
smoking cessation
under
No
(CTH) Care.
Once quit smoking
for minimum 6
months can re-join 1982 Aged
treatment*
released
Act and
Optimal
been
Deficiency is
pharmacological
≤11 μM
No
therapy and best
has
Health
supportive care
Information
of
Yes
of
document
Augmentation therapy with
Department
Prolastin-C or Zameira in addition
This Freedom
to optimal pharmacological
the
treatment and supportive care
by
*Patients should be monitored for
failure to quit smoking, and if relapse
occurs they will lose access to the
intervention
Figure 2 Proposed clinical management algorithm for patients with emphysema and FEV1 <80%
9.
Comparator
The application stated that there are currently no active comparators for augmentation therapy
that modify the progression of emphysema or COPD in patients with A1-PI deficiency. The
comparator for patients with COPD is BSC.
5
Page 5 of 18
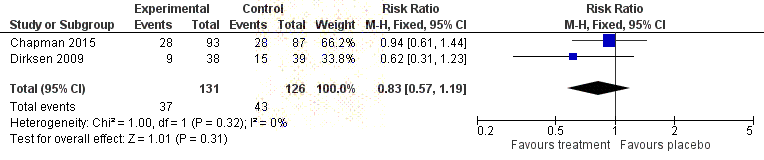
FOI 5155 - Document 5
10. Comparative safety
The application stated that three randomised controlled trials (RCT)s were identified that
evaluated the effectiveness of A1-PI compared to placebo (n=313). Included patients were
relatively homogenous across the included studies, representing ex- or never-smokers with
severe A1-PI deficiency (serum A1 ≤11 µM) and emphysema (forced expiratory volume in
1 second (FEV1) 25% to 80%). The included RCTs were generally well conducted; however,
the method of allocation concealment was poorly reported across all trials. Seventeen single-
arm studies were identified that provided evidence on the safety of A1-PI. Key safety
outcomes were: death due to adverse events, severe adverse events, and discontinuation or
hospitalisation due to adverse events.
The application stated that six deaths occurred in the eligible studies, which included a total
of 899 patients. None of these deaths was reported to be treatment-related. Severe adverse
events were also uncommon, with a median occurrence of 2% in the patient population
the
(range 0%-38%). Discontinuation due to adverse events had a median occurrence of 0.5% in
the patient population (range 0%-12%) across nine studies. Hospitalisation had a median
occurrence of 1.5% in the patient population (range 0%-14%) across four studies.
The application stated that three studies reported safety in patients treated
under with one of the two
Care.
therapies under assessment, Zemaira and PROLASTIN-C. All of these studies found t
(CTH) hat
rates of severe adverse events were unchanged across intervention groups (Figure 3).
Aged
released 1982
Act and
been
has
Health
of
Figure 3 Forest plot indicating the pooled rate of severe adverse events for A1-PI compared to placebo
Information
The application stated that fifteen studies reported any adverse event, with a rate ranging
of
from 0% to 100% and a median of 37%. Differences between the RCTs and observational
studies in the rates of any adverse event may indicate under-reporting in the observational
studies. Dyspnoea and treatment-r
document elated adverse events were also reported. Dyspnoea
occurred after augmentation therapy in 12.5% of the patient population (range 0%-35%).
Events reported by the authors to be treatment-rel
Department ated had a median occurrence of 11% in the
This Freedom
patient population (range 0%-38%).
the
The application stated that overall, it appears that the intervention is safe, with most events
by
being related to the underlying disease.
11. Comparative effectiveness
CT-measured lung density was the primary outcome in two RCTs, and FEV1 was the primary
outcome in one RCT.
No significant differences between A1-PI and placebo were identified in relation to mortality,
exacerbation of COPD, hospitalisation due to COPD exacerbation, quality of life (St.
George's Respiratory Questionnaire), respiratory function (FEV1), exercise capacity
(incremental shuttle walk test) or carbon monoxide diffusion capacity (DLCO). No relevant
data were identified for dyspnoea.
6
Page 6 of 18
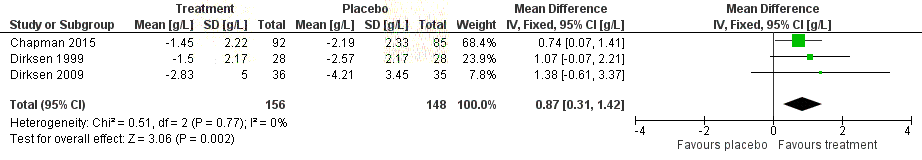
FOI 5155 - Document 5
The only statistically significant difference observed was for CT-measured lung density
(Figure 4), which favoured A1-PI.However, the clinical significance of this difference is
uncertain, as MCIDs for changes in CT-measured lung density have not been established in
the peer-reviewed literature.
Figure 4 Forest plot indicating changes in CT-measured lung density (g/mL) in A1-PI compared to placebo
measured at 24 to 30 months fol ow-up. (Chapman 2015 and Dirksen 1999 reported an annualised rate, whereas
Dirksen 2009 reported the change from baseline at 24 months.)
the
The summary of findings (incorporating both benefits and harms) is shown in Table 2.
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
7
Page 7 of 18
FOI 5155 - Document 5
Table 2 Balance of clinical benefits and harms of A1-PI relative to placebo as measured by the critical patient-
relevant outcomes in the key studies
Outcomes
Risk with
Risk with A1-PI
Relative effect
Participants
Quality of
Comments
(units)
placebo
(95% CI)
(95% CI)
(studies)
evidence
Follow-up
(GRADE)
Uncertain due
Mortality
12 per 1,000
RR 0.35
34 per 1,000
180 (1 RCT)
⨁⨁⨁⨀ to low event
F/U 24 months
(2 to 78)
(0.05 to 2.27)
MODERATE rate, RR subject
to error
Quality of life
MD 0.83 points
Direction
(SGRQ)
lower
favours
-
-
248 (2 RCT)
⨁⨁⨀⨀ placebo; not
F/U 24 to 30
(3.49 points lower
LOW
statistical y
months
to 1.82 points
higher)
significant
Annual
Higher reported RR
Direction
exacerbation
(1.26, 95% CI 0.92
favours
rate
the
-
-
to 1.74), MD (0.36,
257 (2 RCT)
⨁⨁⨁⨀ placebo; not
F/U 24 to 30
95% CI -0.44 to
MODERATE statistical y
months
1.16) in A1-PI group
significant
CT-measured
SMD 0.87 g/L
Direction
lung density
higher
under
-
-
304 (3 RCT)
⨁⨁⨁⨁ favours A1-PI;
Care.
F/U 24 to 30
(0.31 higher to
(CTH)
HIGH
statistical y
months
1.42 higher)
significant
Mortality due to
No reported
treatment-
deaths due to
1982 Aged
related adverse No treatment-related deaths reported
180 (1 RCT)
⨁⨁⨁⨀ treatment-
events
MODERATE
released
related adverse
F/U 24 months
events
Act and
Severe
Direction
adverse events
283 per 1,000
RR 0.83
341 per 1,000
been
257 (2 RCT)
⨁⨁⨁⨁ favours A1-PI;
F/U 24 to 30
(195 to 406)
(0.57 to 1.19)
HIGH
not statistically
months
significant
Health
Discontinuation
has
due to adverse
Direction
of
events
10 per 1,000
RR 0.22
48 per 1,000
248 (2 RCT)
⨁⨁⨁⨀ favours A1-PI;
not statistically
Information
F/U 24 to 30
(2 to 62)
(0.04 to 1.30)
MODERATE
significant
months
of
Hospitalisation
due to adverse
497
events
Median rate 1.4% (range 0.0% t
document o 14.3%)
(4 observational ⨁⨁⨀⨀
F/U 3 to 6
studies)
LOW
-
years
Department
Abbreviations:
F/U = follow-up,
MD = mean dif erence,
RR = relative risk,
SGRQ = St George’s Respiratory Questionnaire,
SMD =
This Freedom
standardised mean dif erence.
GRADE Working Group grades of evidence (Guyatt et al., 2013)
the
⨁⨁⨁⨁
High quality: We are very confident that the true ef ect lies close to that of the estimate of effect.
⨁⨁⨁⨀
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the
by
effect, but there is a possibility that it is substantially dif erent.
⨁⨁⨀⨀
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially dif erent from the estimate of
the effect.
⨁⨀⨀⨀
Very low quality: We have very lit le confidence in the effect estimate: The true effect is likely to be substantially dif erent from
the estimate of effect.
Clinical claim
The clinical claim is that, relative to best supportive care, A1-PI (with either product) slows
disease progression in patients with severe A1-PI deficiency and emphysema. On the basis of
the evidence presented, the contracted assessment stated that A1-PI therapy has uncertain
effectiveness relative to best supportive care, and that relative to placebo, there appear to be
no important differences in safety outcomes associated with A1-PI therapy.
8
Page 8 of 18
FOI 5155 - Document 5
12. Economic evaluation
A cost-utility analysis was undertaken to determine the value of A1-PI in addition to optimal
pharmacological treatment and supportive care (best supportive care).
Table 3 Summary of the economic evaluation
Perspective
This economic evaluation was conducted from the perspective of the Australian health
system. It includes resource use supported by government and patients, along with health
outcomes applicable to the treatment of patients with emphysema due to A1-PI deficiency.
Intervention
Augmentation therapy in addition to optimal pharmacological treatment and supportive care.
Comparator
Best supportive care: optimal pharmacological treatment and supportive care
Type of economic
Cost-utility analysis
evaluation
Sources of evidence
RAPID study, RAPID-OLE study, UK Registry data
the
Time horizon
30-year time horizon in the base case
Sensitivity analyses include a time horizon of 20 years and 40 years
Outcomes
Quality-adjusted life years (QALYs) gained and life-years gained
under
Methods used to
Cohort expected value analysis
(CTH) Care.
generate results
Health states
1.
FEV1≥50% predicted, no lung density decline
2.
FEV1≥50% predicted, slow lung density decline
1982 Aged
3.
FEV1≥50% predicted, rapid lung density decline
4.
FEV
released
1<50% predicted, no lung density decline
5.
FEV
Act and
1<50% predicted, slow lung density decline
6.
FEV1<50% predicted, rapid lung density decline
7.
Lung transplant
been
8.
Dead
Cycle length
1 year
has
Health
Discount rate
5% used for base and 3.5% and 7% sensitivity analyses
of
Software packages used
Microsoft Excel 2010
Information
of
Using a weighted average price for the two A1-PI products, the modelled incremental cost-
effectiveness ratio (ICER) of A1-PI in addition to BSC (relative to BSC alone) was found to
document
be s47(1)(b) per QALY over a time horizon of 30 years. Adopting a modelled time horizon
equivalent to the trial duration (four years) yielded an ICER of s47(1)(b) per QALY
Department
(Table 4). This Freedom
the
Table 4 Incremental cost-effectiveness ratio (1,000-patient cohort)
by
Cost (AU$)
Incremental Effectiveness Incremental ICER (AU$)
cost (AU$)
(QALYs)
effectiveness
Trial period
A1PI augmentation therapy
s47(1)(b)
2,985.3
162.7
s47(1)(b)
Best supportive care
18,531,803
2,822.6
Lifetime
A1PI augmentation therapy
s47(1)(b)
5,826.6
1,301.1
s47(1)
(b)
Best supportive care
37,389,939
4,525.4
Abbreviations:
A1PI = Aplha-1 proteinase inhibitor;
ICER = incremental cost-effectiveness ratio;
QALY = quality-adjusted life year.
9
Page 9 of 18
FOI 5155 - Document 5
The assessment noted that the price paid for the augmentation therapy product is the key
driver of model results (Table 5).
Table 5 Drivers of the economic model
Description
Method/Value
Impact
The average dosing for augmentation therapy is
The base cost of augmentation therapy
s47(1)
Cost of the AT
taken from the RAPID trial and applied to an average assumes a price per 1,000 ml
This
(b)
s47(1)(b)
product
weight of 75.9 kg. The number of vials (rounded to a varies from
per 1,000ml vial. The
whole number) is multiplied by average, high and low estimated ICER varies considerably between
AT product prices.
s47(1)(b) and s47(1)(b) per QALY.
Transition between There were considerable dif erences in transition
A higher number of patients move to the
FEV
between health states for the augmentation therapy FEV1<50 decline states on the BSC arm in
1 and CT
density decline
and BSC arms in the RAPID trials. The economic
RAPID. Movement during the trial period drives
during RAPID drives model assumes movement to no, slow and rapid
economic results. Al owing transition between
clinical benefit
decline tracks during the trial period is sustained for no, slow and rapid tracks after 4 years has
a lifetime.
limited impact on the estimated I
the CER.
In most cases the Gompertz model is the best fit
The specification of the FEV<50 rapid-decline
model had the largest impact on the estimated
Selection of
model to extrapolate survival and this model is used ICER. The use of Lognormal, Generalised
extrapolation model across all non-transplant states. The model is varied Gamma and Weibull m
under odels resulted in the
for the FEV
as part of sensitivity analyses that included use of
Care.
1<50
ICER being 10% more cost effec
(CTH) tive, while use
rapid-decline group the Log-logistic, Lognormal, Weibull, Exponential
of the Exponential model resulted in a 10%
survival
and Generalised Gamma specifications. Large
numbers of patients transition to this state during the decrease in cost effectiveness.
trial period, particularly on the BSC arm.
1982 Aged
Disease management costs in many reviewed
This variation has limited impact as economic
released
COPD economic models were an aggregate of
results are governed by AT product costs. The
Act
proportion of sev
and ere COPD patients who are
Disease
maintenance and acute care costs during flare ups. very severe, assumed to be 74% in the base
management costs The frequency of flare ups was not explicitly
cases, also varied. Similarly, this scenario had
been
for COPD
modelled in this assessment. The Thomas et al.
2014 analysis included acute care proportions for
limited impact on the estimated ICER.
each state. They are varied by 20% for each COPD
Health
state.
has
Abbreviations:
BSC = best supportive care,
COPD = chronic obstructive pul
of monary disease,
CT = computed tomography,
FEV1 = forced
expiratory volume in 1 second,
ICER = incremental cost effectiveness ratio.
Information
13. Financial/budgetary impa
of
cts
The financial impact of the potential listing of A1-PI augmentation therapy is calculated
document
using an epidemiological approach over a five-year period, based on an estimate of the
number of patients eligible for treatment.
Department
This Freedom
Table 6 Estimated financial impact to government from augmentation therapy listing
the
2019
2020
2021
2022
2023
by
Total government costs
AT patients
s47(1)(b)
NBA-supported AT product costs
MBS-supported infusion service delivery
277,422
328,838
381,828
436,429
443,412
Total net costs to governments
s47(1)(b)
Abbreviations:
AT = augmentation therapy,
MBS = Medical Benefit Schedule,
NBA = National Blood Authority.
A key uncertainty is the price of augmentation therapy. Variations in price have a large
impact on both financial and economic attractiveness because of the large contribution of the
augmentation therapy itself to overall resource in the economic model. The proposed price of
PROLASTIN-C is s47(1) per 1,000ml vial and ZEMAIRA s47(1) . An average price of s47(1) is
(b)
(b)
(b)
10
Page 10 of 18
FOI 5155 - Document 5
included, withs47(1) ands47(1) used as high and low bounds in sensitivity analyses. Varying
the prevalence p
(b) roportions
(b) by 10% has a lesser financial impact. Uptake rate also has an
impact. A decrease in year 2022 uptake from 90% to 80% results in a s47(1)(b)
budget
requirement in that year. MSAC noted that the financial estimates were sensitive to
assumptions regarding rates of diagnosis of A1-PI deficiency and non-smoking rates. MSAC
noted advice from the product manufacturers in their pre-MSAC responses that patients
receiving A1-PI are highly motivated to maintain their non-smoking status.
14. Key issues from ESC for MSAC
ESC key issue ESC advice to MSAC
Rarity or under- Alpha1-proteinase inhibitor (A1-PI) deficiency appears to be
diagnosis of
underdiagnosed in the USA, which means it could also be the case in
condition in
Australia. The population may therefore be much larger than the
Australia
estimateds47(1 patients.
the
)(b)
Safety
Overall, it appears that the intervention is relatively safe compared to
placebo, in addition to best supported care. under
Effectiveness
The only statistically significant difference observed was for CT-
(CTH) Care.
measured lung density, which favoured A1-PI therapy compared to
placebo; however, the clinical significance of this difference is
uncertain, as MCIDs for changes in CT-measured lung density have not
1982 Aged
been established in the peer-reviewed literature. No significant
released
differences between A1-PI and placebo were identified in relation to
Act
mortality, exacerbation of COPD, hospitalisation due
and to COPD
exacerbation, quality of life (SGRQ), respiratory function (FEV1),
exercise capacity (incremental s
been huttle walk test) or carbon monoxide
diffusion capacity (DLCO).
has
Health
Costs
Not all relevant costs were captured (e.g. additional A1-PI serum tests,
of
additional IgA tests, IV device, additional consultations).
Information
Population
Trials included patients with a wide range of lung function.
of
Rule of Rescue It is claimed that A1-PI deficiency meets three of the four criteria
warranting the Rule of Rescue. It is unclear whether CT-measured lung
document
density is a sufficiently informative surrogate for judging the Rule of
Rescue criterion of ‘worthwhile clinical improvement’.
This
Department
Potential bias
The small pool
Freedom of researchers and the low frequency of investigator-
initiated tr
the ials mean there is potential for selection and/or reporting bias.
by
ESC discussion
The request is for lifelong intravenous blood augmentation therapy via weekly infusions of
purified human A1-PI (60 mg/kg per week) for the treatment of A1-PI deficiency, also
known as alpha-1 antitrypsin deficiency (AATD). ESC noted that ongoing trials are
investigating optimal dosing regimens (including higher doses). ESC noted the
manufacturers’ claim that successful listing of the blood product in the target population and
setting will lead to slower disease progression compared to best supportive care.
11
Page 11 of 18
FOI 5155 - Document 5
ESC noted that A1-PI deficiency is an inherited genetic condition that results in decreased
circulating, and/or abnormally functioning, A1-PI protein. Severe A1-PI deficiency (defined
as serum levels of A1-PI ≤11 μM) most commonly manifests as emphysema or liver disease.
Prevalence data in Australia are limited. The prevalence of the PiZZ (protease inhibitor,
homozygote Z) allele in Australia, which is identified in the most severely affected patients
(with greatly increased risk of emphysema), is estimated at 1 in 5,584. The prevalence of
PiSZ, which is identified in individuals who produce less A1-PI than normal (and have an
increased risk of emphysema), is estimated at 1 in 841. ESC noted that it is the PiZZ allelle
that contributes to the greatest burden of lung disease in the A1-PI deficient population, but
not all people with PiZZ A1-PI deficiency go on to develop severe emphysema.
ESC noted that the intended population comprises ex-smokers or patients who have never
smoked, who have emphysema and severe A1-PI deficiency (serum A1-PI ≤11 μM). ESC
noted that the contracted assessment estimated that the number of people meeting the criteria
the
for treatment with A1-PI in Australia in 2018 was likely to be s47(1) Considering treatment is
lifelong and not curative, the number of patients being treated is ex
(b)
pected to have a moderate
cumulative increase over time. However, ESC noted that A1-PI appears to be under-
diagnosed in the USA, which means it could also be the case in Australia.
under ESC noted that
(CTH) Care.
there are estimated 80,000–100,000 patients with severe A1-PI deficiency in the USA (Stoller
et al.;
UpToDate).
Aged
A1-PI augmentation therapy is an intervention that can be added to BS
1982 C for patients with
emphysema. ESC noted clinical advice received during the asses
released sment that emphasised the
necessity for patients to maintain a non-smoking status for thi
Act s augment
and ation therapy to be
effective.
been
ESC noted that 17 single-arm studies were included for the evaluation of safety outcomes.
Overall, it appears that the intervention is safe, with most obser
Health ved events judged as being
has
related to the underlying disease. ESC noted that patients with an IgA deficiency are at risk of
of
an anaphylactic reaction.
Information
ESC noted that no studies comparing
of A1-PI augmentation therapy to optimal
pharmacological treatment and supportive care were identified. ESC noted that, because of
the rarity of A1-PI deficiency, clin
document ical trials are often underpowered to detect statistical
differences in outcomes (such as quality of life and mortality). The key studies of A1-PI
therapy have used CT-measured lung density (P
Department D15; 15th percentile lung density) as a
This
primary outcome. It is claimed th
Freedom at CT-measured lung density correlates to markers of lung
health and mortality, and this
the correlation has been used to infer clinical efficacy. PD15 has
been validated as a consistent measure of lung density, specifically in A1-PI deficient
patients, in order to ove
by rcome the challenges of adequately powering a study to detect
significant differences in functional outcomes (such as FEV1) (Parr et al. 2006; Schluchter
et al. 2000). However, ESC noted that minimum clinically important differences (MCID) in
CT-measured lung density for predicting changes in disease progression have not yet been
defined in the peer-reviewed literature.
ESC noted that three randomised controlled trials (RCTs) were identified (RAPID,
EXACTLE and DIRKSEN99) that evaluated the effectiveness of A1-PI therapy compared to
placebo in 313 patients. The studies included ex-smokers or patients who have never smoked,
with severe A1-PI deficiency (serum A1-PI ≤11 µM) and a range of emphysema severity
(FEV1 [forced expiratory volume in 1 second] 25% to 80%). ESC noted that different
12
Page 12 of 18
FOI 5155 - Document 5
primary outcome measures were defined by the investigators: the RAPID and EXACTLE
trials used CT-measured lung density, while the DIRKSEN99 trial used FEV1.
ESC noted that, at 24–30 months, no significant differences between A1-PI augmentation
therapy and placebo were identified across these RCTs in relation to mortality, exacerbation
of chronic obstructive pulmonary disease (COPD), hospitalisation due to COPD
exacerbation, quality of life (St George’s Respiratory Questionnaire; SGRQ), respiratory
function (FEV1), exercise capacity (incremental shuttle walk test) or carbon monoxide
diffusion capacity (DLCO). No relevant data were identified for dyspnoea as a measure of
respiratory function, or the BODE index (BMI, obstruction, dyspnoea, exercise capacity).
The only statistically significant difference observed was for CT-measured lung density,
which favoured A1-PI therapy. However, ESC noted that the clinical significance of this
difference is uncertain, as MCIDs for changes in CT-measured lung density have not been
established in the peer-reviewed literature. However, ESC noted a recent American Thoracic
the
Society conference abstract that has proposed an MCID threshold of –2.89 g/L
(95% CI: -2.59, -3.25; Crossley et al 2018). In this context, one of the product manufacturers
stated that “based on the annual preservation of lung tissue (0.74 g/L/year) demonstrated in
the RAPID trial in favour of A1-PI therapy, the proposed MCID would be ach
under ieved within
(CTH) Care.
3.9 years as compared to an untreated patient.”
ESC noted that the EXACTLE trial reported four methods for measuring CT-measured lung
Aged
density. The assessment report used the 24-month data from the physiol
1982 ogical adjustment
method for comparability with the DIRKSEN99 and RAPID tria
released ls. ESC noted that a
Cochrane review (Gotzsche and Johansen 2016), that included a
Act n aver
and age of the four
methods, yielded almost identical results as the assessment meta-analysis, indicating
concordance of the different methods. been
ESC noted that the comparative effectiveness measured by FEV
Health 1 (showing no statistically
has
significant difference between A1-PI therapy and placebo) was also similar across the
of
assessment meta-analysis and the Cochrane review.
Information
ESC noted that 12 studies reported on t
of he correlation between CT-measured lung density, and
lung function measures (FEV1, KCO gas transfer) and patient-relevant outcomes (mortality
and quality of life). However, ESC
document noted confounding variables, such as differences in
assessing lung density and lung zones, and that the reported correlations were largely cross-
sectional rather than comparing changes in CT-m
Department easured lung density with changes in lung
This
function measures over time. ESC
Freedom noted a meta-analysis (Crossley et al.) reported a
correlation between CT-mea
the sured lung density and FEV1 and KCO gas transfer, although
there was a high degree of heterogeneity across included studies.
by
ESC noted the conclusions of the Assessment Report that, overall, CT-measured lung density
correlates with lung function measures (FEV1 and KCO) and mortality, but findings were
inconsistent regarding correlations between CT-measured lung density and quality of life.
ESC noted the claim that A1-PI therapy meets three of the four criteria warranting Rule of
Rescue. However, it is unclear whether CT-measured lung density is a sufficiently
informative surrogate for the Rule of Rescue criterion of ‘worthwhile clinical improvement’.
ESC noted there is the potential for selection and/or reporting bias in this area of research,
given the small pool of researchers and the low frequency of investigator-initiated trials.
13
Page 13 of 18
FOI 5155 - Document 5
ESC also noted an earlier meta-analysis (COPD 2009; 6(3):177-84) showed A1-PI
augmentation therapy was associated with a 26% reduction in rate of FEV1 decline (absolute
difference 17.9 mL/year; 95% CI 9.6 to 26.1 mL/year) in a subset of patients with baseline
FEV1 of 30–65%. Similar trends were seen in patients with baseline FEV1 of <30% or >65%,
but they were not statistically significant. This 26% treatment effect was used to drive
differences across the A1-PI therapy and BSC arms of the modelled economic evaluation.
ESC provided the following responses to key clinical policy issues:
• Regarding whether there is clinical evidence to support a recommendation for public
funding of A1-PI products – ESC noted that this requires accepting that CT-measured
lung density has been demonstrated to be a surrogate for outcomes known to be clinically
meaningful.
• Regarding potential management criteria – ESC queried whether FEV1 should be added
to the proposed initial eligibility criteria as a more objective measure of emphysema
the
severity. ESC noted that FEV1 25% to 80% reflected the eligibility criteria across the
three identified RCTs, and queried whether this could form the basis for stipulating a
suitable threshold.
under
• Regarding whether there is any material distinction between alpha-1 products current
Care. ly
(CTH)
registered in Australia (Prolastin-C and Zemaira), affecting clinical utility or price level –
ESC noted evidence in the contracted assessment that demonstrated the two agents are
bioequivalent, with 60 mg/kg once weekly regimens yielding equivalent changes in
1982 Aged
trough serum antigenic A1-PI levels. Neither product was found to be cost-effective at the
released
prices currently proposed by the respective manufacturers.
Act and
ESC noted that the results of the modelled economic evaluation were presented in two steps.
The first step outlined cost-effectiveness results for
been the trial period of four years. This length
of follow-up reflects the maximum follow-up of the RAPID trial (Chapman et al. 2015) and
the open-label extension study (RAPID-O
has LE) (McElvaney et a
Health l. 2017). An average
hypothetical cohort of 1,000 patients progresses betw
of een FEV1% and CT-measured lung
density decline states based on results of the trial within a cohort-based semi-Markov model.
Information
Numerical differences in mortality across the A1-PI therapy and BSC arms were taken from
of
the RAPID-OLE and RAPID studies for the first two and four years, respectively
(McElvaney et al. 2017); (Chapman et al. 2015).
document
The efficacy benefit associated with treatment that leads to improvements in patient
morbidity were captured in the model using RAP
Department ID trial data, with the primary analysis
This
being expressed as the increment
Freedom al cost per additional QALY gained. Resource use was
attached to each state using pr
the oposed A1-PI maintenance therapy product costs and MBS
item costs. Australian Refined Diagnosis Related Groups (AR-DRG) costs were applied to
by
the frequency of GP and hospital presentations for UK COPD patients of differing severity
(Thomas et al. 2014) to estimate disease management costs of A1-PI deficiency.
The second step involved extrapolating RAPID transition data over an additional 26 years
(lifetime). It was assumed that transitions between health states with varying rates of CT-
measured lung density decline occurred during the follow-up of the RAPID and RAPID-OLE
studies and that patients stayed on no, slow or rapid decline tracks for the remaining 26 years.
The patient-level data on which the post hoc linear regression analyses were based were
provided to the Assessment Group by the manufacturer that sponsored the RAPID and
RAPID OLE studies.
14
Page 14 of 18
FOI 5155 - Document 5
Mortality data for the remainder of the model’s lifelong time-horizon were based on
observations from 10 years of followed-up patients in the UK AATD registry. A number of
parametric models were fitted to the UK registry data by the Assessment Group to extrapolate
observational data for the lifetime projections.
ESC noted that a range of sensitivity analyses were undertaken to test the robustness of the
results of the modelled economic evaluation. This included changes in baseline distributions
of individuals with emphysema or COPD stratified according to extent of airflow obstruction,
and being mild, moderate, or severe.
ESC noted that most models for COPD health states are stratified by FEV1. However, given
that CT-measured lung density was the primary outcome in the RAPID trial, the model also
incorporated FEV1 to define the health states in the model as well as three levels pf predicted
decline in CT-measured lung density (none, slow or rapid decline) as a driver for mortality.
Patients could move from FEV1>50% to FEV1<50% health states, but not the other way
the
around.
ESC noted clinical advice provided to the Assessment Group that, for the extrapolation after
4 years, the rate of CT-measured lung density decline in A1-PI patients sta
under bilises.
(CTH) Care.
Accordingly, the model assumed that, after the first 4 years of the modelling timeframe,
patients would remain in the no, slow or rapid decline pathways for the remainder of the
modelled timeframe.
Aged
1982
In the pre-modelling studies undertaken by the Assessment Group t
released o extrapolate overall
survival from UK registry with follow-up to 10 years, the Gom
Act pertz func
and tion was found to
have the best fit (lowest AIC statistic) across most subpopulations and, for consistency, was
used in the base case for all subpopulations. ESC
been noted that, whilst this choice was
reasonable, other extrapolation functions of this overall survival curve were more favourable
for the intervention.
has
Health
of
ESC noted that the model was driven by the larger number of patients who are retained in the
Information
FEV1<50% slow decline state, as a result of augmentation therapy. Most incremental life
years saved (LYS) and quality-adjuste
of d life years (QALYs) accrue to the FEV1<50% slow
decline state from the FEV1<50% rapid decline state.
document
ESC noted the economic model yielded base case results well above the threshold usually
considered by MSAC to be acceptably cost-effec
Department tive: with an ICER of s47(1)(b) per QALY
This
for the trial period of 4 years, and
Freedom an ICER of s47(1)(b) per QALY for the lifetime (30 year)
model.
the
ESC noted that the incr
by emental clinical benefit in the model accrues between 5 and 15 years
(i.e. is driven by extrapolation of effects beyond the 4-year trial period). Sensitivity analyses
showed that the cost of A1-PI product is the key driver of the economic model (accounting
for s47(1) of the cost). It is therefore uncertain what price would be acceptably cost-effective.
(b)
ESC noted that, even at the lowest proposed price of s47(1) per 1,000 mL of A1-PI therapy,
the lifetime modelled ICER is
(b)
s47(1)(b)
per QALY. Unit prices that would generate ICERs
within the range usually considered to be acceptable by MSAC are unlikely to be acceptable
to the manufacturers. Consequently, ESC suggested the assessment group be asked to explore
different ‘continuation rule’ scenarios, using the existing model structure, that are evidence-
based and clinically feasible.
15
Page 15 of 18
FOI 5155 - Document 5
For example, what would the ICER impact be if A1-PI therapy was ceased after 4 years (the
trial period), in patients who exhibit a rapid CT-measured lung density decline rate (for
example >2.0 g/L) while on treatment? ESC noted this would require inclusion of CT-
measured lung density scans (at a frequency that would need to be justified) to monitor
response, and therefore need to be added to treatment costs in the model, while being
removed from disease management costs (to avoid double-counting).
When looking at the financial/budgetary impacts, ESC noted that there is no direct estimate
available for the number of Australian patients with COPD with A1-PI deficiency. Estimates
were derived from the prevalence of COPD patients in Australia, the estimated prevalence of
ZZ phenotypes in the USA (adjusted to reflect Australian ethnicities), and the rate of A1-PI
diagnosis using US data. ESC noted that if A1-PI augmentation therapy is funded on the
NPL, current testing rates are likely to increase due to the availability of a treatment option.
ESC noted that the base case estimate of total costs to government wass47(1)(b) million
the
(2019–2023). ESC noted that these estimates are highly sensitive to the price of the products
and were based on the weighted average of the price proposed by each of the two
manufacturers.
under
(CTH) Care.
ESC noted that the financial estimates were also sensitive to assumptions around diagnosis
rates and assumptions regarding the proportion of non-smokers in otherwise potentially
eligible patients, and that higher rates for both of these assumptions are plausible and could
Aged
reasonably be expected to yield financial estimates 2–3 times higher tha
1982 n those presented as
the base case.
released
Act and
ESC noted that the financial estimates are highly sensitive to:
been
• the price of A1-PI therapy;
• assumptions around the proportion of patients with COPD who are diagnosed as A1-
Health
PI-deficient; and
has
• the proportion of potentially eligible patients
of who are assumed to be non-smokers.
Information
ESC suggested the assessment group also undertake additional sensitivity analyses of the
of
financial estimates around the price of A1-PI therapy, that correspond directly to the
‘continuation rule’ scenarios explored in the economic model, noting that, for the scenario
suggested above, this might requir
document e extending the timeframe of the financial analysis to
10 years so that the impact of therapy cessation after 4 years can be captured. If a
‘continuation rule’ is proposed, any additional M
Department BS costs associated with implementing the
This Freedom
rule (e.g. for CT-measured lung density scans, smoking status tests) would need to be
captured in the revised financi
the al estimates.
by
ESC noted that an issue was raised at PASC about whether Indigenous Australians might be
discriminated against if treatment was stopped when a patient continues smoking. However,
ESC noted that PASC had received clinical expert advice that this is a disease mainly
affecting non-Indigenous Australians. It was noted that objective criteria would be needed for
all patients receiving therapy, and that there is a significant opportunity cost for continuing
A1-PI therapy in patients who smoke (as the treatment is rendered entirely ineffective by
smoking).
ESC noted the following key economic and financial policy issues for MSAC:
• The prices proposed by manufacturers do not yield ICERs within the range that is
typically considered to be acceptably cost-effective.
16
Page 16 of 18
FOI 5155 - Document 5
• There is uncertainty surrounding both the primary outcome measure (CT-measured lung
density), and also its correlation with survival, which suggests post-listing data collection
would be warranted – the Australian Patient Registry proposed by one of the companies,
could facilitate this.
• The treatment is high cost (s47(1)(b) per patient per year) for their lifetime, and known to
be ineffective in smokers. Strict requirements would be needed to ensure use is limited to
non-smokers.
• The potential role for other continuation rules for A1-PI therapy could be explored, e.g. in
patients who are not or no longer responding to treatment (after an agreed duration of
treatment, and according to pre-specified, objective criteria) – again, the proposed
Australian Patient Registry could assist with this.
• The potential role for a Risk Sharing Agreement between the NBA and the manufacturers
could be explored to manage the real potential of under-estimation of diagnosis and
treatment rates in the potentially eligible population.
the
• Public funding of A1-PI therapy may result in changes in management; for example,
increased use of prior tests (i.e. capturing test-negative individuals as well as diagnosed
individuals), use of tests to monitor compliance with smoking cessatio
under n, and use of tests
to monitor response to A1-PI therapy. If MBS-funded, these impacts are not c
(CTH) urrentl
Care.y
captured in the financial estimates.
15. Other significant factors
Aged
released 1982
Nil
Act and
16. Applicant’s comments on MSAC’s Public Summary Document
been
CSL Behring is disappointed MSAC did not support A1-PI replacement therapy for the
treatment of A1-PI deficiency with COPD
has . A1-PI deficiency w
Health ith COPD is a life-threatening
and very rare condition with no currently funded dis
of ease-modifying treatment alternatives.
CSL Behring agrees with MSAC that there is a high unm
Information et medical need for patients with
A1-PI deficiency and strong consumer support for funded access, and is pleased that MSAC
of
acknowledged the clear physiological effect of A1-PI therapy on lung density. CSL Behring
maintains that the evidence supporting the benefit of A1-PI therapy is strong in the context of
this rare and slowly progressive di
document sease, noting that it is not feasible to collect survival
outcome data in a clinical trial setting likely to be sufficient to satisfy MSAC’s requirements
in a timely manner. CSL Behring believes there i
Department s a strong basis for applying a broader
This
decision-making framework in thi
Freedom s context, beyond the conventional evaluation approach
used in MSAC’s consideration. C
the SL Behring remains committed to working with the
National Blood Authority and the Jurisdictional Blood Committee to continue to progress the
by
application for timely funded treatment for Australian patients suffering from this devastating
disease.
Grifols is disappointed with the decision by the Medical Services Advisory Committee
(MSAC) not to support purified human alpha1-proteinase inhibitor (A1-PI) for the treatment
of patients with A1-PI deficiency, but is committed to work with the National Blood
Authority (NBA) and other relevant stakeholders, including clinicians and patient
organisations, to ensure that this effective medicine, with a positive impact on survival, will
be made available to those in need and who have the greatest capacity to benefit using
appropriate mechanisms (e.g. Grifols latest generation genetic tools, initiation and
continuation criteria). Grifols welcomes the acknowledgement by the Evaluation Sub-
committee (ESC) that A1-P1 deficiency is a rare disease and that clinical trials for rare
17
Page 17 of 18
FOI 5155 - Document 5
diseases are often underpowered to detect clinically significant outcomes. Furthermore, the
company is keen to work through the cost-effectiveness, albeit acknowledging the current
conventional framework is not well suited to treatments for rare diseases like A1-PI. Indeed,
other factors such as the current lack of clinically effective treatments, clinical need,
seriousness of the disease, the rule of rescue, as well as access and affordability from the
patient perspective and the comparatively small financial implications to the government,
should also be considered when assessing the social value of medicines to treat A1-PI.
17. Further information on MSAC
MSAC Terms of Reference and other information are available on the MSAC Website:
visit the MSAC website
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
18
Page 18 of 18
FOI 5155 - Document 6
Purified human
alpha1-proteinase
inhibitor for the
treatment of alpha1-
proteinase inhibit
the
or
deficiency, leading
to chronic
under
(CTH) Care.
obstructive
pulmonary disease
1982 Aged
released
Act and
been
has
Health
No
of
vember 2018
Information
of
document
MSAC application no. 1530
This Freedom
Department
the
Assessment report
by
Annex to Section D
Page 1 of 11
FOI 5155 - Document 6
CONTENTS
CONTENTS
............................................................................................................................... I
LIST OF TABLES AND FIGURES ............................................................................................................... II
Tables ....................................................................................................................................... ii
Figures ..................................................................................................................................... ii
LIST OF TERMS ............................................................................................................................. III
SECTION D ANNEX ADDITIONAL SENSITIVITY ANALYSIS ......................................................................... 1
Background ..............................................................................................................................
the
1
Sensitivity analysis A – stopping rule ....................................................................................... 1
Sensitivity analysis B – rate of FEV1>50 to FEV1<50 progression ............................................ 5
under
(CTH) Care.
REFERENCES .............................................................................................................................. 7
1982 Aged
released
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
i
Page 2 of 11
FOI 5155 - Document 6
LIST OF TABLES AND FIGURES
TABLES
Table 1
Base transition matrix, augmentation therapy Years 1-4 .................................................. 2
Table 2
Augmentation therapy patient numbers, Years 1-4 (hypothetical cohort of 1000) .......... 2
Table 3
Transition matrix with no and slow decline to rapid progression set to zero,
augmentation therapy Years 1-4 ........................................................................................ 3
Table 4
Augmentation therapy patients, under no progression scenario, Years 1-4,
the
(hypothetical cohort of 1000) ............................................................................................ 3
Table 5
ICER over a life time for stopping and base case (hypothetical cohort of 1000) .............. 4
under
Table 6
Augmentation therapy and best supportive care transition matrices (Years
(CTH) 0-4) ............
Care. 5
Table 7
Differences between AT and BSC annual probabilities of transition (Years 0-4) ............... 6
Aged
released 1982
F
Act
IGURES
and
been
Figure 1
Costs over lifetime for AT arm (1000 cohort) using base and stoppage rule..................... 4
Health
has
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
ii
Page 3 of 11
FOI 5155 - Document 6
LIST OF TERMS
A1PI
Alpha-1 proteinase inhibitor
AT
Augmentation therapy
BSC
Best supportive care
CT
Computed tomography
ESC
Evaluation Sub-Committee
the
FEV1
Forced expiratory volume in 1 second
ICER
Incremental cost-effectiveness ratio
under
(CTH) Care.
QALY
Quality-adjusted life year
1982 Aged
released
Act
and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
iii
Page 4 of 11
FOI 5155 - Document 6
SECTION D ANNEX ADDITIONAL SENSITIVITY ANALYSIS
BACKGROUND
Following review of the Contracted Assessment report for MSAC Application 1530, the Evaluation
Sub Committee (ESC) suggested different ‘continuation rule’ scenarios be explored, using the
existing model structure, that are evidence-based and clinically feasible. Two patient scenarios were
suggested for investigation:
1. Patients who demonstrate limited treatment response to augmentation therapy (AT),
measured with computed tomography (CT) lung density scans.
the
2. Patients that recommence smoking after starting treatment with AT.
under
This document reports the results of additional economic sensitivity analysis for the first sto
Care.ppage
(CTH)
rule only (limited treatment response). The second scenario has not be investigated based on clinical
feedback suggesting patients treated with AT in Australian practice do not recommence smoking (J
1982 Aged
Burdon & P Wark 2018, personal communication, 10 November).
released
Act and
The financial impact of the continuation rules was not investigated
because there is no impact to the
MBS. There are no existing MBS items for urinalysis or CT lung density scanning, and clinical
been
feedback suggests that software needed for CT lung density scanning is not routinely available in
Health
Australian clinical practice (J Burdon & P Wark
has 2018, personal communication, 1 November).
of
Questions were also asked about the use of a non-significan
Information t 26% reduction in FEV1 decline for the
AT arm which was not supported by ra
of ndomised trials outlined in Section B of the assessment. The
rationale for the inclusion of 26% based on the meta-analysis of Chapman et al (2009) is provided,
along with an additional sensitivity an
document alysis if both arms are assumed to have the same FEV1 decline,
or reversed.
This Freedom
Department
SENSITIVITY ANALYSIS A – STOPPING RULE
the
by
Transition probabilities for the economic model are derived from RAPID individual patient data,
outlined by s47G
Patients move between FEV1<50 and FEV1>50 CT-scan lung density
decline states according to what was observed in the four years of RAPID and RAPID OLE for AT
(Chapman et al. 2015, McElvaney et al. 2017). The annual probabilities are provided in Table 1,
which are taken from s47G
Lung density decline is a progressive disease, therefore it could be expected that decline would
continue under AT, albeit at a slower pace compared to best supportive care (BSC). The stoppage
rule would most likely be applied to patients who move from no decline or slow decline to rapid
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
1
Page 5 of 11
FOI 5155 - Document 6
decline tracks for both FEV1 greater or less than 50 (i.e. not no decline to slow decline given the
disease is progressive) even though they are availing AT. The transition from no or slow decline to
rapid decline represents a clinically significant progression of symptoms, suggesting that AT is
ineffective. The annual probabilities for these transitions are outlined in Table 1 and are highlighted
in yel ow. None are higher than s47(1)
s47(1)
(b)
and most between (b)
.
Table 1
Base transition matrix, augmentation therapy Years 1-4
FEV1
>50
FEV1
>50
FEV1
>50
FEV1
<50 FEV1
<50 FEV1
<50
no
slow
rapid
no
slow
rapid
decline
decline
decline
decline
decline
decline
FEV
s47(1)(b)
1>50 no decline
FEV1>50 slow decline
FEV
the
1>50 rapid decline
FEV1<50 no decline
FEV1<50 slow decline
FEV
under
1<50 rapid decline
(CTH) Care.
Source: s47G
Abbreviations: FEV1 = forced expiratory volume in one second
1982 Aged
The numbers of patients in a 1000 hypothetical starting cohort from 0 to 4 years on the AT arm are
released
presented in Table 2. It is evident that most are on the FEV1>50 slow decline and FEV1<50, slow
Act and
decline tracks as the annual probabilities from the key trial reported by CSL direct most patients this
way. Of the 1000, arounds47(1
s47(1
been
)(b)
are in rapid CT decline FEV1>50 and)(b) n rapid CT lung decline FEV1<50
tracks by Year 4. The progressing patients who would cease using AT based on a stopping rule are
has
Health
among these two patient groups.
Information
of
Table 2
Augmentation therapy patient numbers, Years 1-4 (hypothetical cohort of 1000)
of
FEV1
>50
FEV1
>50
FEV1
>50
FEV1
<50,
FEV1
<50,
Years
no
slow
rapid
FEV1
<50,
slow
rapid
Lung t- Cumulative
decline
decline
decline
no decline decline
decline
plant
Death
document
s47(1)(b)
This Freedom
Department
the
by
Abbreviations: FEV1 = forced expiratory volume in one second
Annual probabilities for no and slow decline transition to rapid decline tracks are set to zero to
gauge how many of the rapid decline patients progressed from no decline and slow decline tracks.
The adjusted annual probabilities are presented in Table 3.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
2
Page 6 of 11
FOI 5155 - Document 6
Table 3
Transition matrix with no and slow decline to rapid progression set to zero, augmentation therapy
Years 1-4
FEV1
>50
FEV1
>50
FEV1
>50
FEV1
<50 FEV1
<50 FEV1
<50
no
slow
rapid
no
slow
rapid
decline
decline
decline
decline
decline
decline
FEV
s47(1)(b)
1>50 no decline
FEV1>50 slow decline
FEV1>50 rapid decline
FEV1<50 no decline
FEV1<50 slow decline
FEV1<50 rapid decline
Abbreviations: FEV1 = forced expiratory volume in one second
These probabilities are applied to the starting AT cohort of 1000 and patient number
the s for the 1000
cohort outlined in Table 4 for Years 0-4. It is evident that there are s47(1)
(b)
patients in FEV1>50, rapid
decline, ands47(1)
(b)
in FEV1<50, rapid CT-measured lung decline, tracks by year 4. This represents a
under
different patient number of around s47(1)
(CTH) Care.
(b)
between the base and no progression transition matrices for
rapid decline tracks in Year 4. Based on this calculation, around s47(1)
(b)
patients progress from no and
slow decline lung decline to rapid decline despite being on AT by Year 4. Given the annual
1982 Aged
probabilities of transitioning from no and slow to rapid decline when using AT are relatively low
released
(from CSL individual patient data), this number could be expected.
Act and
Table 4
Augmentation therapy patients, under no progression scenario, Years 1-4, (hypothetical cohort of
been
1000)
FEV1>50 FEV1>50 FEV1>50 FEV1<50, FEV1<50, FEV1<50, Lung
has
Health
Years
no
slow
rapid
no
slow
rapid
transplant Cumulative
decline
decline
decline
decline
of
decline decline -following Death
0
s47(1)(b)
Information
1
of
2
3
document
4
Department
s47(1)(b)
This Freedom
the
Abbreviations: FEV1 = forced expiratory volume in one second
by
The model assumes patients on FEV1>50 no decline, FEV1>50 slow decline, FEV1>50 rapid decline,
FEV1<50 no decline, FEV1<50 slow decline and FEV1<50, rapid decline patients fol ow the same
survival curves regardless of whether they are availing AT or BSC treatment. AT treatment results in
patients moving to different tracks (mainly slow decline) with the adoption of treatment. Stopping
the s47(1)
(b)
AT patients on the rapid track from using AT results in cost savings from avoided AT product
usage and delivery, however, there is no change in the estimate number of QALYs from Year 4
onwards. Patients are assumed to survive the same number of years and have same quality of life
once they are on the rapid track regardless of treatment.
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
3
Page 7 of 11
FOI 5155 - Document 6
The cost saving from avoided AT is significant and presented in Figure 1. Given a year of AT
treatment per patient is more than s47(1)(b)
, stopping s47(1)
(b)
patients who progressed, results in
savings of more than s47(1)(b)
per year for the hypothetical cohort. Additional chest CT scans (e.g.
MBS Items 56301, 56307) are around $300-400 depending on use of contrast, noting that specialised
software is needed to measure lung density that is currently not available in routine clinical practice
in Australia (J Burdon & P Wark 2018, personal communication, 1 November). At $300 per scan, the
additional cost for all s47(1)
(b)
patients in Year 4 (those who haven’t died or had lung transplant surgery)
and all 1000 starting patients is less than $1 million.
s47(1)(b)
the
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
The ICERs for the stopping rule and bas
of e case scenarios are presented in Table 5. It is estimated that
stopping rule ICER is s47(1)(b) compared to s47(1)(b) for the base case, or around 91%. This more
cost-effective ratio broadly reflects th
document e decrease in the number (arounds47(1)
(b)
, or the starting cohort
of 1000) of patients who were deemed to progress from no and slow decline too rapid and stopped
Department
AT.
This Freedom
the
Table 5
ICER over a life time for stopping and base case (hypothetical cohort of 1000)
by
Incremental
Discounted
discounted Effectiveness Incremental
Cost
cost
(QALYs)
effectiveness ICER
Stopping rule
Augmentation therapy
s47(1)(b)
5,826.6
1,301.1
s47(1)
(b)
Best Supportive Care
37,389,939
4,525.4
Base case
Augmentation therapy
s47(1)(b)
5,826.6
1,301.1
s47(1)
(b)
Best Supportive Care
37,389,939
4,525.4
Abbreviations: ICER = incremental cost-effectiveness ratio,
QALY = quality-adjusted life year
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
4
Page 8 of 11
FOI 5155 - Document 6
SENSITIVITY ANALYSIS B – RATE OF FEV1>50 TO FEV1<50 PROGRESSION
The AT arm has a slower rate of FEV1>50 to FEV1<50 progression in the economic model, based on a
s47(1)
(b)
reduction in FEV1 decline derived from the meta-analysis of Chapman et al. (2009) described by
s47G
This assumption is evident in Table 6, with BSC FEV1>50 states having annual
probabilities of transition to FEV
s47(1)
s47(1)
s47(1)
1<50 of (b)
(highlighted in yel ow), compared to (b) for AT (b)
less).
Table 6
Augmentation therapy and best supportive care transition matrices (Years 0-4)
FEV1>50
FEV1>50
FEV1>50
FEV1<50 FEV1<50 FEV1<50
no
slow
rapid
no
slow
rapid
decline
decline
decline
decline
decline
decline
the
BSC
FEV
s47(1)(b)
1>50 no decline
FEV1>50 slow decline
under
FEV1>50 rapid decline
(CTH) Care.
FEV1<50 no decline
FEV1<50 slow decline
FEV
1982 Aged
1<50 rapid decline
AT
released
FEV
Act and
1>50 no decline
FEV1>50 slow decline
been
FEV1>50 rapid decline
FEV1<50 no decline
has
Health
FEV1<50 slow decline
of
FEV1<50 rapid decline
Information
Source: s47G
of
Abbreviations: AT = augmentation therapy,
BSC = best supportive care,
FEV1 = forced expiratory volume in one second
Randomised trials presented in the c
document linical evidence section of the assessment report (e.g. page 66,
Table 35 and Figure 13), indicate however, that a slower rate of FEV1 overall for the placebo arm
Department
than the AT arm, w
This ith a non-statistically significant relative reduction favouring placebo in the meta-
Freedom
analysis of 17%. s45
the
by
s47G
indicated that s45
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
5
Page 9 of 11
FOI 5155 - Document 6
The difference between these annual probabilities for the AT and BSC arms are presented in Table 7.
It is evident that the key differences between the AT and BSC arm annual probabilities are in the no,
slow and rapid decline groups within the FEV1<50 and FEV1>50 patient groupings (highlighted in
yellow), rather than between FEV1>50 and FEV1<50 (in red). These probabilities were provided by
s47(1)(b)
using RAPID study IPD.
Table 7
Differences between AT and BSC annual probabilities of transition (Years 0-4)
FEV1>50
FEV1>50
FEV1>50
FEV1<50 FEV1<50 FEV1<50
no
slow
rapid
no
slow
rapid
decline
decline
decline
decline
decline
decline
s47(1)(b)
Diff = AT-BSC
FEV1>50 no decline
the
FEV1>50 slow decline
FEV1>50 rapid decline
FEV1<50 no decline
under
FEV1<50 slow decline
(CTH) Care.
FEV1<50 rapid decline
Abbreviations: AT = augmentation therapy,
BSC = best supportive care,
FEV1 = forced expiratory volume in one second
Aged
1982
Univariate sensitivity analysis involving changing specific transitions from FEV1 >50 to FEV1<50 does
released
not have a major impact on the ICER. If both AT and BSC arms had
Act FEV
and 1 annual probability declines
of s47(1)(b) , then the
ICER would increase from s47(1)(b)
.
been
s47(1)(b)
has
Health
The base ICER s47(1)(b) was generated using a reduction of s47(1)
s47(1)
(b)
while a (b)
of
reduction resulted in an ICER of s47(1)(b)
. Reversing the FEV
s47(1)(b)
1 decline (i.e.
Information
for AT and s47(1)(b) for BSC) in the CSL Excel model results in an ICER of s47(1)(b) .
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
6
Page 10 of 11
FOI 5155 - Document 6
REFERENCES
Chapman, K, Burdon, J, Pi tulainen, E, Sandhaus, R, Seersholm, N, Stocks, J, Stoel, B, Huang, L, Yao, Z,
Edelman, J & McElvaney, N, 2015. Intravenous augmentation treatment and lung density in
severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial,
Lancet (london, england), 386(9991), pp. 360-368.
Chapman KR, 2009. AT for α1 Antitrypsin Deficiency: A Meta-Analysis. COPD: Journal of Chronic
Obstructive Pulmonary Disease, 6(3), pp. 177-84
CSL Behring, 2017. Schedule 4 proposal supporting the addition of ZEMAIRA to the National Products
List, CSL Behring , Melbourne [Commercial in confidence].
McElvaney, NG, Burdon, J, Holmes, M, Glanvil e, A, Wark, PA, Thompson, PJ, Hernandez, P, Chlumsky,
the
J, Teschler, H, Ficker, JH, Seersholm, N, Altraja, A, Makitaro, R, Chorostowska-Wynimko, J,
Sanak, M, Stoicescu, PI, Pi tulainen, E, Vit, O, Wencker, M, Tortorici, MA, Fries, M, Edelman,
JM & Chapman, KR, 2017. Long-term efficacy and safety of alpha1 proteinase inhibitor
treatment for emphysema caused by severe alpha1 antitrypsin deficiency: an open-label
under
extension trial (RAPID-OLE), Lancet Respir Med, 5(1), pp. 51-60
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
7
Page 11 of 11
FOI 5155 - Document 7
ADDITIONAL SENSITIVITY ANALYSIS (MSAC 1530)
ECONOMIC EVALUATION
Upon request by MSAC, additional sensitivity analysis was conducted around the delivery cost
for alpha-1 antitrypsin augmentation therapy (AT). The cost per vial at the requested
incremental cost-effectiveness ratio (ICER) thresholds of $60,000, $70,000 and $100,000 are
presented in Table 1 (highlighted in yel ow), along with the base case (s47(1)
(b)
) and existing
sensitivity analyses evaluating the impact of a 15% increase (s47(1)
s47(1)
(b)
) or decrease ((b)
) in cost.
Table 1
Sensitivity analysis (cost per vial) for lifetime analysis
AT delivery cost
Incremental cost
Incremental effect
ICER
s47(1)(b)
under the
Abbreviations:
AT = augmentation therapy,
ICER = incremental cost-effectiveness ratio.
FINANCIAL IMPLICATIONS
The budgetary impact associated with the additional sensitivity analysis on the delivery cost
is presented in Table 2. The base case is presented, along with estimated costs at the
corresponding ICER thresholds of $60,000 (Scenario 1, vial cost s47(1)
(b)
, $70,000 (Scenario 2, vial
cost s47(1)
s47(1)
(b)
and $100,000 (Scenario 3, vial cost (b)
Table 2
NET government cost sensitivity analysis (cost per vial)
Projection Year
2019
2020
2021
2022
2023
This document has been released
AT patients
s47(1)(b)
Freedom of Information Act 1982 (CTH)
Base Case
by the Department of Health and Aged Care.
NBA-supported AT product
costs
MBS-supported infusion
service delivery
277,422
328,838
381,828
436,429
443,412
Total costs to government s47(1)(b)
Scenario 1: Vial = s47(1)(b)
NBA-supported AT product
costs
MBS-supported infusion
service delivery
$277,422
$328,838
$381,828
$436,429
$443,412
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
1
Page 1 of 2
FOI 5155 - Document 7
Total costs to government s47(1)(b)
Scenario 2: Vial =s47(1)(b)
NBA-supported AT product
costs
MBS-supported infusion
service delivery
$277,422
$328,838
$381,828
$436,429
$443,412
Total costs to government s47(1)(b)
Scenario 3: Vial =s47(1)(b)
NBA-supported AT product
costs
MBS-supported infusion
service delivery
$277,422
$328,838
$381,828
$436,429
$443,412
the
Total costs to government s47(1)(b)
Abbreviations:
AT = augmentation therapy,
MBS = Medicare Benefits Schedule,
NBA = National Blood Authority.
under
(CTH) Care.
Aged
released 1982
Act and
been
has
Health
Information
of
of
document
This Freedom
Department
the
by
Alpha-1 proteinase inhibitor augmentation – MSAC CA 1530
2
Page 2 of 2
Document Outline

































