FOI 5155 - Document 1
s47G
response to MSAC CA 1530
Purified human alpha1-proteinase inhibitor
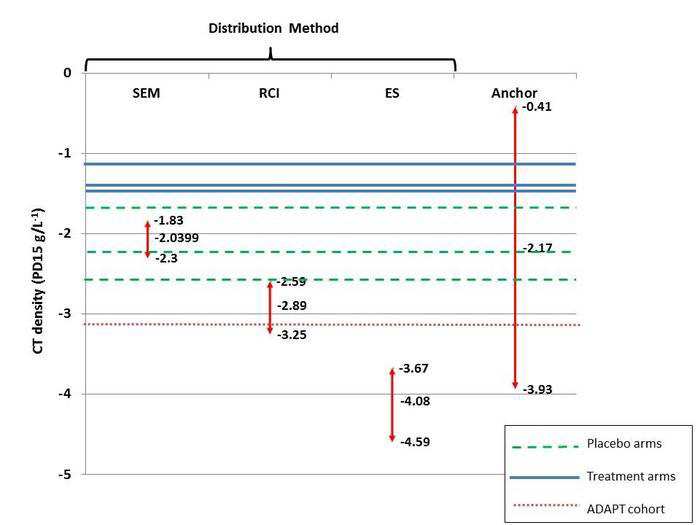
1. “Minimum clinically important differences for the primary outcome in the core randomised
controlled trials (RCTs), i.e. Computed tomography (CT)-measured lung density, are not established
in the literature…” [MSAC CA 1530, p1]
Lung CT densitometry changes have proven to be the most sensitive marker of disease progression in
patients with A1PI deficiency and COPD as compared to pulmonary function tests or quality of life
assessments (Dirksen 2009, Chapman 2015). However, in absence of an established minimum
clinically important difference (MCID) for lung density decline rates, the results seen in the RAPID and
EXACTLE trials may be difficult to interpret. To help address this issue, a group of renowned A1PI
researchers in Birmingham, UK are currently working to establish the MCID based on the CT density
outcomes from the placebo-controlled trials (Dirksen 1999, Dirksen 2009, Chapman 2015). The
researchers recently proposed an MCID of -2.89 g/L (95% CI: -2.59, -3.25) at the American Thoracic
Society conference held in May 2018 (Crossley et al 2018).
Based on the annual preservation of lung tissue (0.74 g/L/year) demonstrated in the RAPID trial in
favor of A1PI therapy, the proposed MCID would be achieved within 3.9 years as compared to an
untreated patient. As the treatment effect was robust and largely consistent between the RAPID and
RAPID OLE trials in the Early Start patients who received 4-years of weekly infusions, a patient
continuously treated with A1PI 60 mg/kg each week can reasonably expect to maintain a reduced rate
of lung density decline well beyond the point at which the proposed MCID has been reached,
demonstrating a worthwhile clinical improvement in this rare and often fatal disease.
2. “No significant differences were observed between A1PI and placebo for the remaining
effectiveness outcomes.” [MSAC CA 1530, p1]
Demonstrating clinical efficacy in A1PI deficiency leading to COPD is challenging. It requires
quantitative documentation of lung function changes in a chronic and slowly progressive process that
may take decades to manifest clinically (Wewers and Crystal 2013). Despite showing a significant
effect on lung density, the RAPID study did not show any statistical signal of efficacy in the secondary
endpoints.
There are several possible reasons for this: First, and importantly, the study was powered to detect
the treatment effect on lung density measures, not changes in pulmonary function tests, diffusion
capacity of carbon monoxide (DLco), Incremental Shuttle Walking Test (ISWT), or St. George’s
Respiratory Questionnaire (SGRQ) scores. The sample size and trial duration reflect those necessary
to demonstrate an effect to slow the annual lung density rates, whereas it has been shown that
significantly more patients followed for periods longer than 2 years would be required to investigate
benefits of A1PI therapy in the secondary endpoints. Furthermore, those estimates are based on the
use of placebo which would be considered unethical for the treatment of A1PI deficiency. Secondly,
the sensitivity of the clinical endpoints to detect change is much lower compared to CT lung density;
EXACTLE, the second largest study in A1PI deficiency, established CT scans and DLco as the most
sensitive measures.
s47G
COMMERCIAL-IN-CONFIDENCE
1
Page 1 of 3
FOI 5155 - Document 1
s47G
response to MSAC CA 1530
Purified human alpha1-proteinase inhibitor
3. “A1PI meets three of the four criteria warranting rule of rescue. It is unclear whether the proposed
service provides worthwhile clinical improvement.” [MSAC CA 1530, p146]
s45
The recent work by Crossley et al to describe
the MCID for CT density decline provides further clinical context for the results seen in the RAPID trial,
and further demonstrates that A1PI offers worthwhile clinical improvement when evaluated across
the appropriate time horizon, noting that A1PI deficiency is a chronic and slowly progressive disease.
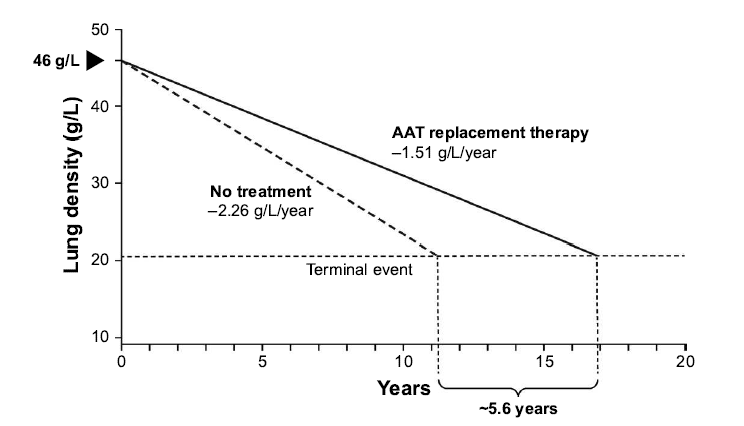
Furthermore, evidence from a post hoc analysis of the RAPID programme suggests a mortality benefit
following A1PI therapy. During the RAPID programme, the time required for progressive emphysema
to develop into respiratory crisis was used to simulate the life-years gained as a result of A1PI therapy.
Respiratory crisis was defined as death, lung transplant or a crippling respiratory condition. Seven
patients withdrew with an average terminal lung density of 20 g/L. Using the average baseline lung
density for all patients (46 g/L) and the rate of decline in lung density in A1PI versus placebo-treated
patients, the projected time to terminal lung density was 16.9 years for those receiving A1PI therapy,
compared with 11.3 years in the placebo group (Figure 1). This indicates a gain in life-years of 5.6 years
with A1PI therapy (McElvaney et al 2017). Although conducted in a small sample size, these data are
supported by results from the National Heart, Lung, and Blood Institute observational study showing
that patients receiving A1PI therapy had a greater survival than those not receiving treatment (Alpha-
1-Antitrypsin Deficiency Registry Study Group, 1998).
Figure 1
Extrapolation of the effect of A1PI replacement therapy on the predicted time to
reach terminal respiratory function in RAPID-RCT.
Source: Chapman et al 2018
International Journal of COPD 18(13): 419-432
No comments on the economic evaluation or financial implications are provided in this response as
Section C, D, E were redacted from the report provided to s47G
due to the commercial in
confidence nature of the material.
s47G
COMMERCIAL-IN-CONFIDENCE
2
Page 2 of 3
FOI 5155 - Document 1
s47G
response to MSAC CA 1530
Purified human alpha1-proteinase inhibitor
REFERENCES
Alpha-1-Antitrypsin Deficiency Registry Study Group (1998). Survival and FEV1 decline in individuals
with severe deficiency of alpha1-antitrypsin. The Alpha-1-Antitrypsin Deficiency Registry
Study Group,
Am J Respir Crit Care Med, 158(1), pp. 49-59.
Chapman, K., Burdon, J., Piitulainen, E., Sandhaus, R., Seersholm, N., Stocks, J., Stoel, B., Huang, L.,
Yao, Z., Edelman, J. & McElvaney, N. (2015). Intravenous augmentation treatment and lung
density in severe Alpha-1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-
controlled trial,
Lancet 386(9991), pp. 360-368
Chapman, K. R., Chorostowska-Wynimko, J., Rembert Koczulla, A., Ferrarotti, I., & McElvaney, N. G.
(2018). Alpha 1 antitrypsin to treat lung disease in alpha 1 antitrypsin deficiency: recent
developments and clinical implications.
Int J Chron Obstruct Pulmon Dis 13: 419–432.
Crossley, D., Subramanian, D., Stockley, R. A., & Turner, A., M. (2018). Proposal and validation of a
minimal clinically important difference (MCID) for annual pulmonary CT density decline.
American Journal of Respiratory and Critical Care Medicine 197:A3905
Dirksen, A., Dijkman, J. H., Madsen, F., Stoel, B., Hutchison, D. C., Ulrik, C. S., Skovgaard, L.T., Kok-
Jensen, A.,Rudolphus, A., Seersholm, N., Vrooman, H. A., Reiber, J. H., Hansen, N.C.,
Heckscher, T., Viskum, K. & Stolk, J. (1999). A randomized clinical trial of alpha(1)-antitrypsin
augmentation therapy,
Am J Respir Crit Care Med 160 (5 Pt 1), pp. 1468-1472.
Dirksen, A., Piitulainen, E., Parr, D.G., Deng, C., Wencker, M., Shaker, S.B. & Stockley, R.A. (2009).
Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-
antitrypsin deficiency.
Eur Respir J 33(6), pp. 1345-1353.
McElvaney, N.G., Burdon, J., Holmes, M., Glanville, A., Wark, P. A., Thompson, P. J., Hernandez, P.,
Chlumsky, J., Teschler, H., Ficker, J. H., Seersholm, N., Altraja, A., Makitaro, R., Chorostowska-
Wynimko, J., Sanak, M., Stoicescu, P. I., Piitulainen, E., Vit, O., Wencker, M., Tortorici, M. A.,
Fries, M., Edelman, J. M & Chapman, K. R. (2017). Long-term efficacy and safety of alpha1
proteinase inhibitor treatment for emphysema caused by severe alpha1 antitrypsin
deficiency: an open-label extension trial (RAPID-OLE), Lancet Respir Med, 5(1), pp. 51-60.
Wewers, M. D., & Crystal, R. G. (2013). Alpha-1 Antitrypsin Augmentation Therapy. COPD: Journal of
Chronic Obstructive Pulmonary Disease 10(S1): 64-67
s47G
COMMERCIAL-IN-CONFIDENCE
3
Page 3 of 3
FOI 5155 - Document 1.1
12
45678
9
4
5
7
656
595
!"
#
$""
!%
&!%'()
&(
*+
,-
+.
/&0
1-
+.
&
/#
2
3
45
/0
6*)
7#
!8%'
295
:
0
$%
;
<!
=!%9%
!%
=%
#
6>?@?ABC
BDE
FBC
G
EBHG
?D
?I
B
G
DG
JBC
4C
G
DG
KBC
C
L
J@?>H
BDH
7G
I
IM>MDKM
N4
7O
I
?>
DDPBC
6PC
J?DB>L
4
7MDAG
HL
7MKC
G
DM
;Q
=#
!""5
(+)
;Q
$8R#
%
%*)
SQ
/Q
$
!T5
(U)
/Q
&Q
8#
%#
1V
+/;/
!W
)
X%
9#
"
(
4!"Y
5
"
6
#
%<)
6
#
%<)
X%
'
Z
%<'!)
*S!(5
;#
R(
4!"Y
5
)
;#
R()
X%
'
Z
%<'!)
U28%<
[
%9"
<
!%
X%
)
\8%
]5
^R
4!"Y)
6
#
%<)
X%
'
Z
%<'!)
1/;/
!W
)
\8%
]5
^R
4!"Y
5
)
6
#
%<)
X%
'
Z
%<'!Q
4?>>MA@?DEG
D_
BPH
`?>a
A
MJBG
C
8
EG
BDBb
K>?AAC
MLcdD`Ab
DMH
S
!%5
-
=!Y8
'
!!<#
Y(
3
=:
'%"
!
#
(
"
R%
8"'
"
Y#
#
(
!8
!
"8#
%
"9#
5
S%'!
"'
Y5
R!
=!%
#
!5'
#
5
"
3
S=":
!W
/5
Y
!%
/8<%
!%
#
Y(
3
//:
!
""""
5
!#
!%
!W
5
8%<
'%"
(
'5
%Q
/%
&=[
;
W
!#
=
'%"
(
e!85
'
Y#
!9
'
!#
5
#
(
!W
'%"
(
%<"
%(
Y#
9
"
9
%<
8%#
%
5
%
5
Y
Q
#
#
e!
#
!<%
"'
!'"
W
!#
Y#
!Y!"
%<
%
&=[
;V
%5
(
%!#
%'
'
"
#
R8
!%
!'Q
f
!
'
#
%
%'
95
'
%
&=[
;
W
!#
=
'%"
(
'5
%
%
Y
%
"
e
//;
8"
%<
R!
#
!<%
"'
!'"Q
"
e!85
'
5
#
W
(
W
!W
//)
%'
!#
!
'%
W
(
!"
Y
%
"
e
"
<%
W
%
'5
%
e!
(
%'
%
#
9%
!%Q
&
!'"-
g!#
'
"
#
R8
!%
!')
"
8'
"
e#
"!8<
#
Y!#
'
%
%'
"
%'#
'
'9
!%
!W
=
'%"
(
3
"
"8#
'
R(
+.
#
%
5
!
%
V
;+.
<
2:
R"5
%
%'
e
%%85
%<Q
"
e#
%
8"'
!
5
85
&=[
;
8"
%<
U
9#
!%"
!W
'
"
#
R8
!%
!'-
"
%'#
'
#
#
!#
!W
"8#
%
3
$]&:
)
#
5
R5
%<
%'h
3
S=[
:
)
%'
W
"
^
3
]$:
Q
g!#
%!#
!')
%(
YY#
"
#
Y!#
'
%%85
=
'%"
(
%<
e
#
5
9
%<
%
g]i+
"
"8#
'
%
5
"
e
!8
hY!"8#
!
%
%
#
9%
!%
3
Y5
R!
#
":
e#
#
9
e'Q
&=[
;
e"
%
95
'
'
8"
%<
6
#
%<
//
!!#
Q
%
"
e!
'
#
9'
e!
!#
!#
=
"%"
Y5
8"
5
"
#
g]i+
"8#
%
"
!9#
5
"
#
(#
"
e#
'%
W
'Q
/%%85
"5
!Y
g]i+
3
5
":
e"
5
85
'
%'
!Y#
'
e
#
"Y
9
%%85
=
'%"
(
%<
%'
!Y#
'
R(
5
%#
#
<#
""
!%
%5
("
"Q
S"85
"-
g
<8#
!%
58"
#
"
&=[
;
%'
#
!%W
'%
%
#
95
"Q
!%W
'%
%
#
95
"
W
#
!
%!#
!'
%!Y""'
!"
!W
$]&
%'
S=[
Q
7
9%
Y#
!Y!"'
&=[
;
"!85
'
!#
<
%
W
#
!
9#
(
!W
!'")
"
#
"!%R5
!
Y#
!Y!"
&=[
;
W
!#
=
'%"
(
"
0
*Q
j,<
2
"
"
"
''5
"
W
#
!
#
'
"
#
R8
!%
!'")
%'
"
5
e
%
!%W
'%
%
#
95
"
!W
%!#
!'Q
=!%5
8"
!%-
Y#
!Y!"'
&=[
;
W
!#
=
'%"
(
%
Y
%
"
e
//;
"
0
*Q
j,<
2Q
i5
8"
%
h""
!W
"
%
Y
%
"
8%'#
"8#
9
5%
e!85
'
%'
#
Y
'
'%"
(
'5
%)
e
(
R
!%
%'
!%
W
!#
//
8<%
!%
#
Y(Q
Page 1 of 2
FOI 5155 - Document 1.1
12
3
5637
8
597
2
3
6
2
3
2
7
157
2
93
! "#$%&'()
'*+,
-*&.
/
0!
0!"$$,1112
"!
3
450"6
2
47
806
09!
":!
/
5
Page 2 of 2

FOI 5155 - Document 3.1
Page 1 of 48

FOI 5155 - Document 3.1
Page 2 of 48
FOI 5155 - Document 3.1
Page 3 of 48

FOI 5155 - Document 3.1
Page 4 of 48

FOI 5155 - Document 3.1
Page 5 of 48

FOI 5155 - Document 3.1
Page 6 of 48

FOI 5155 - Document 3.1
Page 7 of 48

FOI 5155 - Document 3.1
Page 8 of 48

FOI 5155 - Document 3.1
Page 9 of 48

FOI 5155 - Document 3.1
Page 10 of 48

FOI 5155 - Document 3.1
Page 11 of 48

FOI 5155 - Document 3.1
Page 12 of 48
Document Outline













