Final report for DVA Human Research Ethics Committee:
Study title: Switching medicines in the Veteran population (ref: E005/009)
Principal researcher:
s 47F
PhD student
c/o Quality Use of Medicines and Pharmacy Research Centre,
University of South Australia
GPO Box 2471, Adelaide SA 5001
Phone: s 47F
Email: s 47F
Outcome of completed research:
Since December 1994, a brand substitution policy has operated on the Pharmaceutical
Benefits Scheme (PBS) and Repatriation Pharmaceutical Benefits Scheme (RPBS), the
nationally funded schemes for medicine subsidy in Australia. Patients can receive a brand
or generic product other than the one prescribed if products are bioequivalent and the
prescription is not marked “substitution not permitted”. Ideally patients should remain on
the same product following initial brand substitution; however there is no limit to the
number of brand substitutions. There are concerns that multiple product changes occur,
with the potential to confuse patients and compromise the quality use of medicines
(QUM). Increased use of generics in Australia is encouraged; however, the brand
substitution policy has not been evaluated since implementation.
This research evaluated implementation of the brand substitution policy from Australia’s
National Medicines Policy perspective, by studying the frequency of brand substitution for
government subsidised medicines and the extent of switching between products by
cohorts of individuals. Administrative claims data for government subsidised medicine
dispensings on the RPBS were used.
The first study in this research characterised brand substitution using the example of
simvastatin, a medicine for which brand substitution was first possible in 2004. Trends in
the rate of brand substitution and the extent of brand substitution per patient were
examined. Over 90% of patients in this analysis had two or less switches; only 9% had
multiple switches. Simvastatin patients with multiple switches were more likely to have
had more prescribers, dispensing pharmacies and original prescriptions than other
patients (p < 0.0001)
The second study of the research further characterised implementation of the brand
substitution policy using six medicine examples. Trends in the rate of brand substitution
for these medicines were examined and the number of brand substitutions per
prescription form and per patient identified. While switching occurred for all products,
analysis of individual prescriptions showed that 92% of prescription forms had the same
product supplied on each repeat dispensing. Analysis by patient showed over 80% of
patients either had no switches or only a single brand substitution, confirming the results
of the first study. Patients with multiple switches were more likely to receive a product with
a brand premium at their initial dispensing.
To complete the brand substitution picture, the final study of the research examined the
extent of brand substitution for all medicines used by a cohort of patients on multiple
medicines. Amongst this cohort, 83% of patients either had no switches or only a single
brand substitution during follow-up for all medicines received. Patients at risk of having
multiple brand substitutions had more prescription medicines, hospital admissions,
dispensing pharmacies, prescribers and longer fol ow-up than other patients (p < 0.0001).
Progress report for DVA HREC – “Switching medicines in the veteran population”
The overall conclusion from the findings of these studies is that the minimum pricing policy
and brand substitution are implemented in the manner intended. They facilitate consumer
access to cheaper medicines without resulting in multiple switches for over 80% of
patients. Initiatives to reduce multiple switching amongst patients identified as being at
increased risk of having multiple switches would further support implementation of the
brand substitution policy.
Conference presentations arising from this research:
A number of conference presentations have reported results of this research. Al
conference abstracts and presentations were reviewed and approved by DVA (Medication
Management Section) prior to presentation. References to the conference proceedings
are listed below, along with a copy of the presentation abstract:
Kalisch L, Roughead E, Gilbert A. Brand switching following patent expiry of simvastatin
and introduction of generic alternatives. In: s 47F E and s 47F C, editors. Proceedings of
the joint meeting of the Australasian Society of Clinical and Experimental
Pharmacologists and Toxicologists (ASCEPT) and the Australasian Pharmaceutical
Science Association (APSA); Melbourne; December 2005. [poster presentation]
Legislation to allow brand substitution by pharmacists at the point of dispensing
was introduced in 1994. Since then use of generic medicines has increased;
however the degree of switching between dif erent brands and generics is
unknown. It has been suggested that switching brands and generics can confuse
patients and lead to potential harm.
This study aimed to identify the rates of switching between dif erent brands and
generics of simvastatin from one dispensing to another.
Simvastatin was chosen as the study drug because a generic alternative became
available in November 2004 (prior to patent expiry in July 2005), allowing
comparison of drug use prior to and post availability of generics. Other statins do
not yet have bioequivalent brands available for substitution. Prescription claims
data for dispensing of simvastatin on the Repatriation Pharmaceutical Benefits
Scheme were analysed.
Preliminary results revealed that between November 1st 2003 and February 28th
2005 542,018 simvastatin prescriptions were dispensed to 110,049 persons. 57%
of patients were male. In the year prior to the availability of generic versions of
simvastatin (November 1st 2003 – October 31st 2004), simvastatin was dispensed
to 43,792 patients, of which 860 (2%) switched at least once. The rate of switching
was one switch per 237 simvastatin prescriptions dispensed. In the first four
months post generic simvastatin availability (three bioequivalent brands available),
38,953 patients were dispensed simvastatin, of which 3,196 (8.2%) switched
brands at least once, with a rate of one brand switch per 35 prescriptions
dispensed.
Future analysis of the data wil assess the influence of switching pharmacy on the
rate of brand switching, as wil the influence of switching prescriber. The influence
of patient characteristics such as age and gender on switching wil be determined.
Rates of switching after July 2005, when 10 simvastatin brands were available,
wil be compared with earlier switching rates.
Kalisch L, Roughead E, Gilbert A. Switching between brand name and generic medicines:
the example of simvastatin. In: Day R and s 47F R, editors. National Medicines
Symposium 2006. Quality Use of Medicines: Balancing beliefs, benefits and harms;
Canberra; June 2006. [oral presentation]
Since introduction of the brand substitution policy to the Pharmaceutical Benefits
Scheme (PBS) in 1994, use of generic medicines and the number of generics
available has increased. In 2005 when over 400 generics were available, 56% of
PBS prescriptions were dispensed at the patient co-payment price, compared to
2
Progress report for DVA HREC – “Switching medicines in the veteran population”
17% in 1994. There is potential for patient confusion and harm when brands and
generics are switched, particularly if switching is common. A small Australian study
found that 56% of patients do not understand the dif erence between brand and
generic names of medicines. Currently, the extent of switching between brand and
generic medicines is unknown. This study aimed to identify simvastatin switching
rates and associated factors.
The first simvastatin generic was PBS listed in November 2004. Switching rates
were compared pre and post generic simvastatin availability. A switch was
identified if different brands or generics were dispensed consecutively. Analyses
were conducted using Repatriation Pharmaceutical Benefits Scheme prescription
claims data.
Prior to generic availability when two non-substitutable brands were available,
there was one switch per 279 prescriptions dispensed. This was most commonly
associated with the use of a dif erent prescription (87% of switches), rather than a
repeat of the same prescription. The switching rate on individual prescriptions was
one switch per 1,773 dispensings. Following introduction of the first generic the
overall switching rate increased to one switch per 14 dispensings. Of these
switches, 24% coincided with dispensing at a dif erent pharmacy to the previous
and 70% were associated with the use of a dif erent prescription. When individual
prescriptions were followed the switching rate was lower, with one switch per 38
dispensings. In 21% of these cases, the switch coincided with a change in
pharmacy at which the medicine was dispensed.
The introduction of a simvastatin generic appears to have increased switching.
Further work wil identify switching rates after August 2005 when ten simvastatin
brands and generics were available.
Kalisch L, Roughead E, Gilbert A. Switching between brand name and generic medicines
- are there multiple switches per prescription? In: s 47F A and s 47F R, editors. Annual
conference of the Australasian Pharmaceutical Science Association; Adelaide; December
2006. [oral presentation]
Introduction: Since December 1994, the brand substitution policy has operated
on the Pharmaceutical Benefits Scheme (PBS) and Repatriation PBS (RPBS).
Patients can receive a brand or generic product other than the one prescribed if
the products being substituted are bioequivalent and the prescriber hasn’t
specified that substitution cannot occur. Currently, there is no limit to the number
of brand substitutions on repeats of an individual prescription. Doctors have
expressed concern that patients may receive a different product each time their
prescription repeats are dispensed, which has the potential to confuse patients.
The Australian Divisions of General Practice has suggested that a limit of one
switch per prescription should be enforced. We aimed to identify the number of
switches per prescription for a range of medicines and to determine the number of
different products supplied on each prescription.
Methods: Al RPBS prescription claims between January 1st 2001 and February
28th 2006 were identified for enalapril, ramipril, atenolol, simvastatin, citalopram,
metformin and omeprazole. Original prescriptions with five repeats and all supplies
dispensed were included. Switches were identified if a dif erent brand or generic
product was supplied on consecutive repeat dispensings. The number of switches
and the number of different products supplied per prescription was calculated.
Results: 535,942 original prescriptions were included. 491,398 (92%) had no
switches on repeats and 37,513 (7%) had only one switch. Only 1% of all
prescriptions had more than one switch identified on repeats, and in most cases
only two dif erent products were supplied. Only seven of the 563,677 prescriptions
studied had a dif erent product supplied on each repeat.
Conclusion: Most prescriptions have the same product supplied on each repeat,
and if switches occur in most cases there is only one switch per prescription.
3
Progress report for DVA HREC – “Switching medicines in the veteran population”
Multiple switches per prescription are uncommon and multiple different products
are rarely supplied on repeats of the same prescription.
Kalisch L, Roughead E, Gilbert A. Brand substitution or multiple switches per patient? An
analysis of pharmaceutical brand substitution in Australia. In: Abstracts of the 23rd
International Conference on Pharmacoepidemiology and Therapeutic Risk Management;
Quebec City, Canada; August 2007. Pharmacoepidemiology and Drug Safety 2007;
16(S2): S55-S56. [oral presentation]
Background: In Australia, when prescriptions are dispensed, brand substitution
can occur if products are bioequivalent and the prescription is not marked
“substitution not permit ed”. Ideally patients should remain on the same product
following the initial brand substitution; however there are concerns that multiple
switches occur. Generics are sold under trade names rather than the generic drug
name and product appearance may differ. Multiple substitutions may cause
confusion due to the change in product name and appearance. The generics
market is growing; however the extent of multiple switching is unknown.
Objectives: To identify whether brand substitution or multiple switching occurs.
Methods: Department of Veterans’ Affairs pharmacy claims data for atenolol,
citalopram, enalapril, metformin, omeprazole and ramipril were analysed. Claims
were included for patients with ≥4 supplies of the same medicine each year from
2001 to 2005. Switches were identified if dif erent products were dispensed
consecutively within 60 days. Brand substitution was defined as one switch, or no
more than 2 switches more than a year apart over the 5 year period. Multiple
switching was defined as use of ≥3 products in 365 days, use of 2 products and ≥4
switches in 365 days, >10 switches over 5 years, and/or use of ≥5 products over 5
years.
Results: 36,538 episodes of medicine dispensings were identified, representing
33,892 patients. 14,779 patients (44%) had no switches and 9,321 (28%) met
brand substitution criteria for each medicine used. 6,168 patients (18%) had
multiple switches for at least one medicine, and over the 5 years 2,722 had more
than one multiple switching episode. Multiple switches were most commonly
identified as use of ≥3 products in 365 days (3,799 patients, 62% of multiple
switchers) or use of 2 products and ≥4 switches in 365 days (2,295 patients, 37%
of multiple switchers).
Conclusions: Most patients using medicine over 5 years (71%) either did not
switch, or demonstrated brand substitution. 18% of patients had multiple switches.
Future research should identify the people, places and specific medicines in which
multiple switching is likely to occur, so that it can be avoided in patients at risk of
confusion and adverse outcomes.
Kalisch L, Roughead E, Gilbert.
Pharmaceutical brand substitution in Australia: identifying
risk factors associated with having multiple brand substitutions. In: s 47F J and s 47F R,
editors. National Medicines Symposium 2008. QUM: what does it really mean for you?
The science, the policy and the practice; Canberra; May 2008. [oral presentation]
Background: The brand substitution policy has operated on the PBS since
December 1994. Anecdotal reports have highlighted the potential for confusion if
brand substitution occurs multiple times, particularly for the elderly and people
using multiple medicines. Factors associated with having brand and generic
products substituted multiple times are unknown.
Aims: To identify factors associated with having multiple brand substitutions and
to determine the frequency with which people have multiple brand substitutions for
multiple medicines.
Methods: A retrospective cohort study was conducted using Department of
Veterans’ Affairs administrative claims data from June 1st 2005 to August 31st
2006. The analysis was limited to people with the opportunity for brand substitution
4
Progress report for DVA HREC – “Switching medicines in the veteran population”
of two or more medicines. “Switches” were identified for each medicine if different
brand or generic products were supplied at consecutive dispensings. The total
number of switches for each medicine dispensed to a patient was determined.
Multivariate analysis was conducted to identify factors associated with multiple
switches.
Results: 84,040 people were included. On average, they received 11 prescription
medicines. 49% of people received the same product throughout follow-up for
each medicine and 34% had a single brand substitution. 17% had multiple
switches for one or more medicine; however, only 3% of all patients had multiple
switches for more than one medicine. Independent risk factors associated with
having multiple switches were increasing number of hospital admissions,
prescription medicines, co-morbidities, prescribers and dispensing pharmacies;
and living in an aged-care facility or major city.
Conclusions: Most patients do not have multiple brand substitutions of their
medicine, even when all medicine use is considered. This is the first study to
identify risk factors associated with having multiple brand substitutions. Quality use
of medicines interventions targeting individuals with these risk factors could
minimise the potential for patient confusion as a result of multiple brand changes.
Publications arising from this research:
Work from this research has been presented in the following peer reviewed publications:
Kalisch LM, Roughead EE, Gilbert AL. Do pharmacists adhere to brand substitution
guidelines? The example of simvastatin. Journal of Pharmacy Practice and Research
2007; 37(4): 292-294.
Kalisch LM, Roughead EE, Gilbert AL. Pharmaceutical brand substitution in Australia –
are there multiple switches per prescription? Australian and New Zealand Journal of
Public Health 2007; 31(4): 348 – 52.
Kalisch LM, Roughead EE, Gilbert AL. Brand substitution or multiple switches per patient?
An analysis of pharmaceutical brand substitution in Australia. Pharmacoepidemiology and
Drug Safety 2008; 17(6): 620 – 25.
This research also formed the basis for a PhD thesis authored by the s 47F
s 47F
).
Copies of these peer reviewed publications and the PhD thesis are appended for
reference.
Events of significance (e.g. adverse outcomes) that have occurred during
the study:
No adverse events of significance have occurred during the study.
Maintenance and security of records:
Security of records has been maintained at all times during the progress of this study. The
study was conducted as part of the Veterans’ MATES project. Well defined security
guidelines exist for the Veterans’ MATES project and regular security audits are
conducted. As outlined in the Veterans’ MATES project management plan, all personal
information has been stored and analysed at the “Protected” security level, as defined in
the Protective Security Manual and DVA security manual. Re-coded, de-identified data
were used for the study. The re-coded data were not removed from the secure area of
QUMPRC.
5
Progress report for DVA HREC – “Switching medicines in the veteran population”
Compliance with the approved proposal and protocol:
An amendment to the original research proposal was submitted to DVA HREC for
consideration at its April 2006 meeting and was endorsed by DVA HREC. No changes
have been made to the research protocol since this amendment was endorsed by DVA
HREC. The research was conducted in accordance with the approved research proposal
and protocol.
Compliance with any conditions of approval:
No special conditions of approval were set for this research.
6
Pharmaceutical brand substitution
in Australia
Lisa Marie Kalisch
BPharm (Hons)
A thesis submitted in fulfilment of the requirements for the degree of
Doctor of Philosophy
Sansom Institute
School of Pharmacy and Medical Sciences
Division of Health Sciences
University of South Australia
March 2008
Table of contents
List of figures.......................................................................................................................vi
List of tables .......................................................................................................................vii
Abbreviations used throughout the text ...........................................................................ix
Summary..............................................................................................................................xi
Statement of originality ....................................................................................................xiii
Acknowledgement .............................................................................................................xiv
Communications arising from this thesis ........................................................................ xv
Chapter 1: Introduction ...................................................................................................... 1
Chapter 2: Australia’s National Medicines Policy - the medicines framework in
Australia ............................................................................................................................... 4
2.1 Australia’s National Medicines Policy ......................................................................... 4
2.1.2 Quality, safety and efficacy of medicines ................................................................. 7
2.1.4 A responsible and viable medicines industry in Australia ...................................... 11
2.2 Challenges for the supply and subsidy of medicines locally and internationally... 13
2.2.1 Supply and demand economic theory – an overview.............................................. 13
2.2.2 Pharmaceutical policies to influence demand ......................................................... 16
2.2.2(i) Prescription cost-sharing ................................................................................. 17
2.2.2(ii) Formularies ..................................................................................................... 17
2.2.2(iii) Generic substitution policies ......................................................................... 18
2.2.2(iv) Prescribing feedback, education and guidelines ............................................ 18
2.2.2(v) Prescribing budget restrictions........................................................................ 19
i
2.2.3 Pharmaceutical policies to influence supply ........................................................... 19
2.2.3(i) Medicines licensing and regulatory approval .................................................. 19
2.2.3(ii) Reference pricing policies .............................................................................. 19
2.2.3(iii) Cost effectiveness pricing policies and economic evaluation ....................... 20
2.3 Policies influencing supply and demand, implemented to ensure continued access
to medicines in Australia via the PBS and RPBS ........................................................... 20
2.3.1 Pricing and listing of PBS medicines...................................................................... 22
2.3.2 Patient co-payments ................................................................................................ 22
2.3.2(i) Safety Net provisions....................................................................................... 23
2.3.3 The minimum pricing policy................................................................................... 24
2.3.3(i) Brand substitution by pharmacists................................................................... 24
2.3.3(ii) Bioequivalence of brand and generic products............................................... 25
2.3.4 The therapeutic group premium policy ................................................................... 26
2.4 Co-payments, the minimum pricing policy and therapeutic group premium
policy: influencing supply and demand on the PBS and RPBS..................................... 27
Chapter 3: PBS policies to influence supply and demand: what is the impact on
achieving the National Medicines Policy objectives?...................................................... 28
3.1 Review of the literature on PBS patient co-payments .............................................. 28
3.1.1 International studies of the effects of increasing patient co-payments ................... 33
3.1.2 Patient co-payments - some conclusions................................................................. 35
3.2 Review of the literature on the PBS minimum pricing policy and brand
substitution policy .............................................................................................................. 36
3.2.1 International studies of brand substitution .............................................................. 47
3.2.2 Brand substitution policies - some conclusions ...................................................... 56
3.3 Review of the literature on the PBS therapeutic group premium policy ............... 57
3.3.1 International studies of reference pricing policies .................................................. 61
3.3.2 Therapeutic group premium and reference pricing policies – some conclusions ... 63
3.4 PBS policies to influence supply and demand – overall conclusions....................... 63
3.4.1 Gaps in the evidence base: the minimum pricing and brand substitution policies
and their potential influence on QUM ............................................................................. 64
Chapter 4: Research design .............................................................................................. 67
4.1 Introduction.................................................................................................................. 67
4.2 Research aims............................................................................................................... 67
ii
4.3 Study design.................................................................................................................. 68
4.4 Study population and data source.............................................................................. 70
4.4.1 DVA treatment cards and level of entitlement to DVA subsidised health services 70
4.4.2 Characteristics of DVA treatment card holders ...................................................... 70
4.4.3 DVA administrative claims data ............................................................................. 71
4.4.3(i) Pharmacy data.................................................................................................. 72
4.4.3(ii) Private hospital admissions data..................................................................... 72
4.4.3(iii) Medical and allied health data ....................................................................... 72
4.4.4 Appropriateness and relevance of DVA administrative claims data for use in the
present research ................................................................................................................ 73
4.5 Preview of studies included in this thesis................................................................... 73
4.6 Ethical approval........................................................................................................... 74
Chapter 5: Brand substitution of government subsidised medicines in Australia: the
example of simvastatin ...................................................................................................... 75
5.1 Introduction.................................................................................................................. 75
5.2 Aims............................................................................................................................... 77
5.3 Methods......................................................................................................................... 77
5.3.1 Identification of switches ........................................................................................ 77
5.3.2 Statistical analysis ................................................................................................... 79
5.4 Results ........................................................................................................................... 80
5.4.1 Rate of switching..................................................................................................... 80
5.4.1(i) Rate of switching: Naïve and stable simvastatin users .................................... 81
5.4.2 Patients with and without switches ......................................................................... 82
5.5 Discussion ..................................................................................................................... 84
5.6 Conclusions................................................................................................................... 86
Chapter 6: Analysis of the extent of brand switching for six medicines: atenolol,
citalopram, enalapril, metformin, omeprazole and ramipril......................................... 87
6.1 Introduction.................................................................................................................. 87
6.2 Aims............................................................................................................................... 88
6.3 Methods......................................................................................................................... 89
6.3.1 Methods: Part 1 – Background analysis.................................................................. 90
iii
6.3.2 Methods: Part 2 - The extent of brand switching on repeats of individual
prescriptions; a “prescription-level” analysis................................................................... 91
6.3.2(i) Identification of dispensings for individual prescriptions ............................... 91
6.3.2(ii) Identification of switches for the prescription-level analysis ......................... 92
6.3.3 Methods: Part 3 - Number of brand switches per patient; a “patient-level” analysis
.......................................................................................................................................... 92
6.3.3(i) Brand substitution status of patients ................................................................ 93
6.3.3(ii) Statistical analysis........................................................................................... 93
6.4 Results ........................................................................................................................... 94
6.4.1 Results: Part 1 – Background analysis .................................................................... 94
6.4.1(i) Major PBS listing changes for the study medicines ........................................ 94
6.4.1(ii) Rate of switching ............................................................................................ 94
6.4.2 Results: Part 2 - The extent of brand switching on repeats of individual
prescriptions; a “prescription-level” analysis................................................................... 97
6.4.2(i) Number of switches per prescription ............................................................... 97
6.4.3 Results: Part 3 - Number of brand switches per patient; a “patient-level” analysis99
6.4.3(i) Brand substitution status of patients ................................................................ 99
6.4.3(ii) Comparison of multiple switchers and non-switchers.................................. 101
6.4.3(iii) Comparison of multiple switchers and brand substitution patients............. 102
6.4.3(iv) Brand substitution status and product received at initial dispensing ........... 103
6.5 Discussion ................................................................................................................... 104
6.5.1 Rate of switching between brand and generic products........................................ 104
6.5.2 The extent of brand switching on repeats of individual prescriptions; the
“prescription-level” analysis .......................................................................................... 105
6.5.3 The number of brand switches per patient; the “patient-level” analysis............... 106
6.5.4 Strengths and limitations of the study................................................................... 108
6.6 Conclusions................................................................................................................. 110
Chapter 7: The extent of brand substitution for patients using multiple medicines . 111
7.1 Introduction................................................................................................................ 111
7.2 Aims............................................................................................................................. 111
7.3 Methods....................................................................................................................... 112
7.3.1 Study period and study medicines......................................................................... 112
7.3.2 Patient inclusion criteria........................................................................................ 112
iv
7.3.3 Identification of switches for each included medicine.......................................... 115
7.3.4 Identification of patient characteristics associated with multiple switches .......... 115
7.3.5 Statistical analysis ................................................................................................. 117
7.4 Results ......................................................................................................................... 117
7.4.1 Multivariate analysis: comparison of patients with multiple switches and patients
with no switches ............................................................................................................. 118
7.4.2 Multivariate analysis: comparison of patients with multiple switches and patients
with a single brand substitution (but no medicines with multiple switches) ................. 120
7.4.3 Multivariate analysis: comparison of patients with multiple switches for two or
more medicines and patients with no switches during follow-up .................................. 121
7.5 Discussion ................................................................................................................... 123
7.6 Conclusions................................................................................................................. 127
Chapter 8: Discussion and conclusions.......................................................................... 128
8.1 Patients at risk of having multiple brand substitutions ......................................... 129
8.1.1 Strategies to avoid multiple brand substitutions ................................................... 130
8.2 Continued and future evaluations of the minimum pricing and brand substitution
policies............................................................................................................................... 133
8.3 Overall conclusions .................................................................................................... 135
References......................................................................................................................... 137
Appendix 1.1 – Publication arising from this thesis ..................................................... 152
Appendix 1.2 – Publication arising from this thesis ..................................................... 156
Appendix 1.3 – Publication arising from this thesis ..................................................... 162
Appendix 4.1 - Department of Veterans’ Affairs administrative claims datasets ..... 169
v
List of figures
Figure 1.1 - Example of the brand substitution policy: simvastatin ...................................... 2
Figure 2.1 - Interdependence of the four objectives of Australia's National Medicines
Policy ..................................................................................................................................... 5
Figure 2.2 - Supply and demand curve ................................................................................ 15
Figure 2.3 - Increasing Government cost of PBS subsidy................................................... 21
Figure 2.4 - Major PBS and RPBS co-payment changes .................................................... 23
Figure 3.1 - Simvastatin packaging: appearance of different brand and generic products.. 65
Figure 5.1 - Simvastatin switching rate ............................................................................... 80
Figure 5.2 - Switching rate: naive and stable users of simvastatin...................................... 81
Figure 5.3 - Prescription dispensed at consecutive dispensings when switches occurred... 83
Figure 5.4 - Simvastatin prescriptions written for five repeats with all six supplies
dispensed post-generics: number of different brand or generic products dispensed per
prescription .......................................................................................................................... 83
Figure 6.1 - Trend in the rate of brand substitution: atenolol .............................................. 95
Figure 6.2 - Trend in the rate of brand substitution: citalopram.......................................... 95
Figure 6.3 - Trend in the rate of brand substitution: enalapril ............................................. 96
Figure 6.4 - Trend in the rate of brand substitution: metformin .......................................... 96
Figure 6.5 - Trend in the rate of brand substitution: omeprazole ........................................ 97
Figure 6.6 - Trend in the rate of brand substitution: ramipril .............................................. 97
Figure 7.1 - Identification of study cohort ......................................................................... 114
vi
List of tables
Table 3.1 - Multiple brand and generic products: different trade names for simvastatin.... 65
Table 5.1 - Simvastatin: timeline of PBS/RPBS listing of brand and generic products...... 76
Table 5.2 - Simvastatin: time between consecutive dispensings ......................................... 78
Table 5.3 - Patients dispensed simvastatin: factors associated with being a multiple
switcher compared to a non-switcher .................................................................................. 82
Table 5.4 - Patients dispensed simvastatin: factors associated with having multiple
switches compared to one or two switches .......................................................................... 82
Table 6.1 - Study medicines ................................................................................................ 89
Table 6.2 - Time between consecutive dispensings for the study medicines ...................... 91
Table 6.3 - Prescriptions with and without switches ........................................................... 98
Table 6.4 - Number of products dispensed on prescriptions with multiple switches .......... 98
Table 6.5 - Gender, age and duration of follow-up for patients included in Part 3 of the
study..................................................................................................................................... 99
Table 6.6 - Brand substitution status of patients included in Part 3 of the study .............. 100
Table 6.7 - Rate ratio (95% confidence interval) of brand substitution status comparisons
for patients using each medicine........................................................................................ 100
Table 6.8 - Factors associated with being a multiple switcher compared to a non-switcher
........................................................................................................................................... 101
Table 6.9 - Factors associated with being a multiple switcher compared to having a single
brand substitution............................................................................................................... 102
Table 6.10 - Product supplied at initial dispensing and number of switches during follow-
up ....................................................................................................................................... 103
vii
Table 7.1 - PBS and RPBS medicines eligible for inclusion............................................. 113
Table 7.2 - Excluded PBS/RPBS items ............................................................................. 114
Table 7.3 - Included patients.............................................................................................. 117
Table 7.4 – Patients with multiple switches: number of medicines with multiple switches
........................................................................................................................................... 118
Table 7.5 - Multivariate comparison of patients with multiple switches for one or more
medicine and patients with no switches for any medicine................................................. 119
Table 7.6 - Multivariate comparison of patients with multiple switches for one or more
medicine and patients with a single brand substitution for one or more medicines .......... 120
Table 7.7 - Multivariate comparison of patients with multiple switches for two or more
medicines and patients with no switches for any medicine ............................................... 122
viii
Abbreviations used throughout the text
ABS
Australian Bureau of Statistics
ACE
Angiotensin Converting Enzyme
ADEC
Australian Drug Evaluation Committee
ADGP
Australian Divisions of General Practice
ADRAC
Adverse Drug Reactions Advisory Committee
AMA
Australian Medical Association
APAC
Australian Pharmaceutical Advisory Council
ARTG
Australian Register of Therapeutic Goods
ASGC
Australian Standard Geographic Classification
AUC
Area Under the Curve
CI
Confidence Interval
Cmax
Maximum plasma concentration
DVA
Department of Veterans’ Affairs
GP
General Practitioner
H2
Histamine-2
HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
HMO
Health Maintenance Organisation
HMR
Home Medicines Review
IRSD
Index of Relative Socio-economic Disadvantage
NPS
National Prescribing Service
OECD
Organisation for Economic Co-operation and Development
OTC
Over the Counter
P3
Pharmaceuticals Partnerships Program
PBAC
Pharmaceutical Benefits Advisory Committee
PBPA
Pharmaceutical Benefits Pricing Authority
PBS
Pharmaceutical Benefits Scheme
ix
Abbreviations used throughout the text – continued
PHARM
Pharmaceutical Health and Rational Use of Medicines
PIIP
Pharmaceutical Industry Investment Program
PPI
Proton Pump Inhibitor
PSA
Pharmaceutical Society of Australia
QUM
Quality Use of Medicines
RCT
Randomised Controlled Trial
RPBS
Repatriation Pharmaceutical Benefits Scheme
SD
Standard Deviation
SHPA
Society of Hospital Pharmacists of Australia
SSRI
Selective Serotonin Re-uptake Inhibitor
TGA
Therapeutic Goods Administration
WHO
World Health Organisation
x
Summary
Since December 1994, a brand substitution policy has operated on the Pharmaceutical
Benefits Scheme (PBS) and Repatriation Pharmaceutical Benefits Scheme (RPBS), the
nationally funded schemes for medicine subsidy in Australia. Patients can receive a brand
or generic product other than the one prescribed if products are bioequivalent and the
prescription is not marked “substitution not permitted”. Ideally patients should remain on
the same product following initial brand substitution; however there is no limit to the
number of brand substitutions. There are concerns that multiple product changes occur,
with the potential to confuse patients and compromise the quality use of medicines (QUM).
Increased use of generics in Australia is encouraged; however, the brand substitution
policy has not been evaluated since implementation.
The studies in this thesis evaluated implementation of the brand substitution policy from
Australia’s National Medicines Policy perspective, by studying the frequency of brand
substitution for government subsidised medicines and the extent of switching between
products by cohorts of individuals. Administrative claims data for government subsidised
medicine dispensings were used.
In the first and second chapters of the thesis, Australia’s National Medicines Policy and the
systems for subsidy and supply of medicines in Australia are described. The concepts of
supply and demand for medicines are introduced, and PBS policies designed to influence
supply and demand for medicines, including the brand substitution policy, are discussed.
The potential impact of these policies on achieving QUM is discussed in Chapter 3 and
gaps in knowledge regarding implementation of brand substitution are identified. In
Chapter 4 suitable research methods and data sources within which to address the research
gaps are identified.
xi
The first study in this thesis, reported in Chapter 5, characterised brand substitution using
the example of simvastatin, a medicine for which brand substitution was first possible in
2004. Trends in the rate of brand substitution and the extent of brand substitution per
patient were examined. Over 90% of patients in this analysis had two or less switches; only
9% had multiple switches. Simvastatin patients with multiple switches were more likely to
have had more prescribers, dispensing pharmacies and original prescriptions than other
patients (p < 0.0001)
The study in Chapter 6 further characterised implementation of the brand substitution
policy using six medicine examples. Trends in the rate of brand substitution for these
medicines were examined and the number of brand substitutions per prescription form and
per patient identified. While switching occurred for all products, analysis of individual
prescriptions showed that 92% of prescription forms had the same product supplied on
each repeat dispensing. Analysis by patient showed over 80% of patients either had no
switches or only a single brand substitution, confirming the results reported in Chapter 5.
Patients with multiple switches were more likely to receive a product with a brand
premium at their initial dispensing.
To complete the brand substitution picture, the study in Chapter 7 examined the extent of
brand substitution for all medicines used by a cohort of patients on multiple medicines.
Amongst this cohort, 83% of patients either had no switches or only a single brand
substitution during follow-up for all medicines received. Patients at risk of having multiple
brand substitutions had more prescription medicines, hospital admissions, dispensing
pharmacies, prescribers and longer follow-up than other patients (p < 0.0001).
In Chapter 8, implementation of the brand substitution policy from the National Medicines
Policy perspective is discussed. The overall conclusion from the findings of the studies
reported in this thesis is that the minimum pricing policy and brand substitution are
implemented in the manner intended. They facilitate consumer access to cheaper medicines
without resulting in multiple switches for over 80% of patients. Initiatives to reduce
multiple switching amongst patients identified as being at increased risk of having multiple
switches would further support implementation of the brand substitution policy.
xii
Statement of originality
I declare that this thesis presents work carried out by myself and does not incorporate
without acknowledgement any material previously submitted for a degree or diploma in
any university. To the best of my knowledge it does not contain any materials previously
published or written by another person except where due reference is made in the text. All
substantive contributions by others to the work presented, including jointly authored
publications, is clearly acknowledged.
Lisa Kalisch
Date
xiii
Acknowledgement
I thank my PhD supervisors, Libby Roughead and Andrew Gilbert for their support along
this journey. Your encouragement, advice and feedback is greatly appreciated. In particular
I thank Libby for her guidance and encouragement, and for reading (and re-reading!)
numerous drafts of papers and this thesis.
I thank the Department of Veterans’ Affairs for supporting this research with a PhD
scholarship and for providing access to the administrative health claims data used in this
research. I thank everyone at QUMPRC for their friendship and support along the way. In
particular, I thank Emmae Ramsay for her statistical advice and assistance.
Thanks to the people who encouraged me to undertake this PhD in the first place, in
particular Lisa Spurling and my parents. Thanks mum and dad for your support and
encouragement, and for always being so proud of everything that I do. Thanks also to Paul
and Lindsay for your support from the other side of the world. And most importantly,
thankyou John for your support and encouragement, and for just being there.
xiv
Communications arising from this thesis
Work from this thesis has been presented in the following publications and conference
presentations:
Peer reviewed publications
Kalisch LM, Roughead EE, Gilbert AL. Do pharmacists adhere to brand substitution
guidelines? The example of simvastatin. Journal of Pharmacy Practice and Research 2007;
37(4): 292-294.
Kalisch LM, Roughead EE, Gilbert AL. Pharmaceutical brand substitution in Australia –
are there multiple switches per prescription? Australian and New Zealand Journal of Public
Health 2007; 31(4): 348 – 52.
Kalisch LM, Roughead EE, Gilbert AL. Brand substitution or multiple switches
per patient? An analysis of pharmaceutical brand substitution in Australia.
Pharmacoepidemiology and Drug Safety 2008; 17(6): 620 – 25.
See Appendix 1 for copies of these publications.
xv
Conference presentations
Kalisch L, Roughead E, Gilbert A. Brand and generic substitution in Australia: are patients
switched between products multiple times? Pharmacoepidemiology and Drug Safety 2007;
16(S2): S55 – S56.
Kalisch L, Roughead E, Gilbert A. Switching between brand name and generic medicines -
are there multiple switches per prescription? In: Davey A and Milne R, editors. Annual
conference of the Australasian Pharmaceutical Science Association; Adelaide; 2006.
Kalisch L, Roughead E, Gilbert A. Switching between brand name and generic medicines:
the example of simvastatin. In: Day R and Boyd R, editors. National Medicines
Symposium. Quality Use of Medicines: Balancing beliefs, benefits and harms; Canberra;
2006.
Kalisch L, Roughead E, Gilbert A. Brand switching following patent expiry of simvastatin
and introduction of generic alternatives. In: Davis E and Sobey C, editors. Proceedings of
the joint meeting of the Australasian Society of Clinical and Experimental Pharmacologists
and Toxicologists (ASCEPT) and the Australasian Pharmaceutical Science Association
(APSA); Melbourne; 2005.
Seminar presentations
Kalisch L, Roughead E, Gilbert A. Brand and generic substitution in Australia: are patients
switched between products multiple times? Presented to UniSA Vice Chancellor Peter Høj,
UniSA Pro Vice Chancellor (Health Sciences) Robyn Mc Dermott and travel scholarship
donor Mr Roy Schulz. Presentation given on 10th July 2007 at the Quality Use of
Medicines and Pharmacy Research Centre, UniSA.
Kalisch L, Roughead E, Gilbert A. Switching between brand name and generic medicines:
the example of simvastatin. Presented at Sansom Institute Research Colloquium:
Influencing medicines policy and medicine use through research; held at the University of
South Australia on August 4th 2006.
xvi
CHAPTER 1
Introduction
The Pharmaceutical Benefits Scheme (PBS) and Repatriation Pharmaceutical Benefits
Scheme (RPBS) are nationally funded schemes for medicine subsidy in Australia. Patients
pay a fixed co-payment for subsidised medicines; the remainder of the cost is met by the
Australian government. In 1990, the minimum pricing policy was introduced to the PBS
and RPBS, and since then only the cheapest brand(s) or generic product(s) for each
medicine are available to patients at the co-payment price.1 A brand substitution policy was
introduced in December 1994 allowing brand substitution of bioequivalent products by
pharmacists at the time of dispensing, provided the prescriber hadn’t specified that brand
substitution could not occur.2,3 All brand and generic products of a medicine available for
PBS and RPBS subsidy are listed in the Schedule of Pharmaceutical Benefits. Products
with the indicator of bioequivalence (a superscript “a” next to the product name) may be
substituted (Figure 1.1). Price premiums for brands costing more than the cheapest
product(s) are shown in the Schedule of Pharmaceutical Benefits, and must be paid in
addition to the standard co-payment (see Figure 1.1).
The minimum pricing policy allows medicine manufacturers to set the price for their
product above the maximum subsidised price and sends a price signal to patients regarding
the cost of their medicines.1 The brand substitution policy facilitates use of cheaper
products, by giving consumers the opportunity to respond to the price signal associated
with more expensive brands of medicine.1 When the brand substitution policy was planned,
it was intended to facilitate substitution of cheaper generic medicines for patients who had
been prescribed brand name medicines which attracted a brand premium.1 Ideally patients
should remain on the same product following the initial brand substitution; however,
legislation does not prevent multiple brand substitutions per patient. In the years since
introduction of the brand substitution policy there have been anecdotal reports and
1

brand substitutions per patient occur; and to identify the people and situations in which
multiple brand substitutions are most likely to occur. Administrative claims data for
medicine dispensings will be used to achieve these aims.
This thesis begins with an overview of Australia’s National Medicines Policy and a
description of medicines subsidy and supply in this context.
3
CHAPTER 2
Australia’s National Medicines Policy - the medicines
framework in Australia
In this chapter Australia’s National Medicines Policy, the framework governing medicines
and their use in Australia, is described. The challenges of meeting the policy goals are
discussed and an overview of the economic theory of supply and demand is provided as a
basis for discussing factors influencing the subsidy and use of medicines. The chapter
concludes with a discussion of policies influencing supply and demand of medicines in
Australia.
2.1 Australia’s National Medicines Policy
In 1987, in recognition of the fact that formal policies were required to ensure and maintain
access to medicines of high quality, safety and efficacy and to ensure the rational use of
medicines, the World Health Organization (WHO) drafted guidelines for the development
of national drug policies.15 WHO describes a national drug policy as:
− “a commitment to a goal and a guide for action. It expresses and prioritises
the medium- to long-term goals for the pharmaceutical sector, and identifies
the main strategies for attaining them. It provides a framework within which
the activities of the pharmaceutical sector can be coordinated. It covers both
the public and the private sectors, and involves all the main actors in the
pharmaceutical field.”16
In 1989 only 14 countries had a national drug policy and by 1999 this had increased to 66
countries.16 Australia was one of the first developed countries to implement a national drug
policy,16 which is referred to in Australia as the National Medicines Policy.
Australia’s National Medicines Policy was formally implemented in 2000;14 however, a
major component of the National Medicines Policy, the Pharmaceutical Benefits Scheme
(PBS), was implemented in the 1950s.17 Other components of the policy were also
developed many years prior to the formal introduction of the National Medicines
4
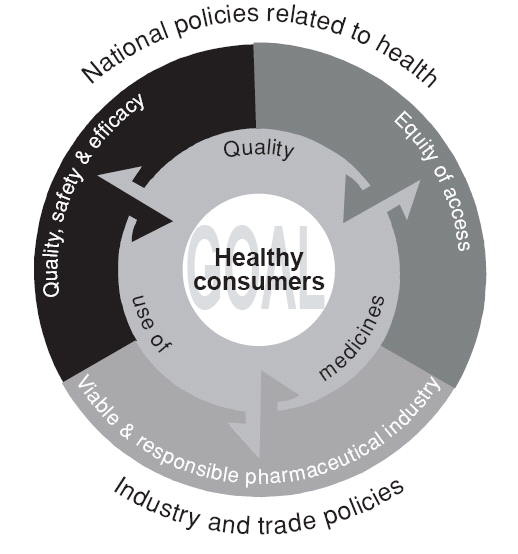
Policy.17-19 The overall aim of Australia’s National Medicines Policy is to “meet
medication and related service needs, so that both optimal health outcomes and economic
objectives are achieved.”14 To achieve this overall aim, the policy focuses on four central
objectives:
•
“timely access to the medicines that Australians need, at a cost individuals and the
community can afford;
•
medicines meeting appropriate standards of quality, safety and efficacy;
•
quality use of medicines; and
•
maintaining a responsible and viable medicines industry.”14
The four objectives of Australia’s National Medicines Policy are “interdependent”19 – that
is, achievement of each objective and the overall goal of the National Medicines Policy
requires each of the other objectives to be fulfilled at the same time. For example, quality
use of medicines (QUM) cannot be achieved if the access objective is not met and people
cannot afford to buy their medicines.19 If the quality, safety and efficacy objective is not
met, then it would be impossible to achieve QUM.19 Maintaining a viable medicines
industry in Australia helps ensure a reliable medicines supply and the development of new
medicines.14 The interdependence of the four objectives of the National Medicines Policy
is illustrated in Figure 2.1.
Figure 2.1 - Interdependence of the four objectives of Australia's National Medicines Policy
(Reproduced from: Commonwealth of Australia. The national strategy for quality use of medicines.
Plain English edition. Canberra: Department of Health and Ageing; 2002.)
5
In line with the WHO guidelines for developing national drug policies, there are programs
and frameworks in place to support achieving each of Australia’s National Medicines
Policy objectives. These are discussed in detail in the next sections of this chapter.
2.1.1 Timely access to medicines at a cost individuals and the community
can afford
Access to medicines not only involves access to an adequate and reliable supply of
medicine, it also involves the ability of individuals and the community to pay for
medicines. The National Medicines Policy states that “cost should not constitute a
substantial barrier to people’s access to medicines they need”.14 The Pharmaceutical
Benefits Scheme (PBS) and the Repatriation Pharmaceutical Benefits Scheme (RPBS), the
government subsidised schemes for supply of medicines in Australia, help to achieve this
part of the access aim.
The PBS commenced in 195017 in response to availability of medicines such as penicillin
and concerns that the majority of Australians would be unable to afford them.20 Both sides
of parliament agreed that the Commonwealth Government had a responsibility to ensure
that Australians had affordable access to medicines.17 When the PBS started, it covered
139 “lifesaving and disease preventing” medicines, available to Australians free of
charge.17,20 In 2007, over 600 medicines21 available as 2,800 different products3 were
subsidised through the PBS. Medicines available for subsidy under the PBS are listed in
the Schedule of Pharmaceutical Benefits,a which is updated monthly.2
The Repatriation Pharmaceutical Benefits Scheme (RPBS) was implemented prior to the
PBS, in 1919.17 Veterans of the first World War and Boer War were eligible for benefits
and could receive free of charge most medicines available in Australia at the time.17 The
format of the RPBS remained largely unchanged until 1983, when benefits were restricted
to medicines available on the PBS plus an extra list of medicines specific to the needs of
veterans.17 Initially only six medicines were included on this "extra" list;17 however the
RPBS has now grown to include over 150 medicines.2
All Australian citizens and permanent residents, as well as visitors from countries with
which Australia has a reciprocal healthcare agreement (namely the United Kingdom, New
Zealand, Finland, Ireland, Italy, Malta, the Netherlands and Sweden) are eligible to receive
a The current Schedule of Pharmaceutical Benefits can be accessed on the internet from:
http://www.pbs.gov.au/html/healthpro/publication/list (last accessed January 17th 2008)
6
medicines subsidised on the PBS.2 RPBS medicines are limited to eligible Department of
Veterans' Affairs (DVA) treatment card holders, who include Australian defence force
veterans and eligible dependants such as widows or widowers. Patients eligible to receive
RPBS medicines can also receive all PBS subsidised medicines.22
There are two benefit categories for medicines subsidy on the PBS - general beneficiaries
and concession beneficiaries. Concession beneficiaries are those people who hold a
government Pension Concession Card, a Commonwealth Seniors Health card, a
government issued Health Care Card or a DVA treatment card.2 General beneficiaries are
all other patients eligible to receive PBS medicines.2 Co-payments differ for general
patients and concession patients. In 2008, concession patients paid $5.00 per PBS
prescription; the general co-payment was up to $31.30 for each PBS prescription, with the
remainder of the cost subsidised by the Australian government.23
2.1.2 Quality, safety and efficacy of medicines
The second objective of Australia’s National Medicines Policy aims to ensure that
medicines manufactured and supplied in Australia are of high quality, safety and efficacy.
This objective is supported by the legislative framework of the Therapeutic Goods Act
1989; which is administered by the Therapeutic Goods Administration (TGA).18,24,25 All
therapeutic goods must be registered or listed on the Australian Register of Therapeutic
Goods (ARTG) before they can be sold in Australia.24,25 Therapeutic goods manufactured
solely for export from Australia must also be included in the ARTG. A therapeutic good is
defined as:
− “a product for use in humans that is used in, or in connection with:
− preventing, diagnosing, curing or alleviating a disease, ailment, deficit or
injury; or,
− influencing, inhibiting or modifying a physiological process; or,
− testing the susceptibility of persons to a disease or ailment; or,
− testing for pregnancy; or
− the replacement or modification of parts of the anatomy.”24
Therapeutic goods may be listed or registered on the ARTG depending on the ingredients
included in the product, its dosage form, and the claims made by the manufacturer
regarding the therapeutic efficacy of the product.24 Listed medicines are considered to be
“low risk” products; most commonly they are the types of medicines patients select for
7
themselves without requiring the input of a health professional, e.g. vitamins.24 The safety
and quality of listed products is assessed by TGA before they can be supplied in
Australia.24 Efficacy of listed medicines is not assessed by TGA; however, it is a legal
requirement that proof of efficacy can be provided if it is requested.24 Listed medicines are
available without a prescription and can be differentiated from registered medicines by an
“AUST L” number on the product label.24
Registered therapeutic goods are generally considered to be higher risk products than listed
medicines.24 They are the types of products which tend to have, or require, some level of
health professional involvement in their sale or use, e.g. medicines sold over the counter in
a pharmacy and prescription only medicines.24 There are two types of registered
medicines: low risk registered products are non-prescription medicines which are available
in a pharmacy and which may require advice from a pharmacist for use, e.g. oral
decongestants and over the counter pain relievers.24 High risk registered products are
available only on prescription.24 Registered medicines can be distinguished from listed
medicines by an “AUST R” number on the product label.24
There is a well defined process for the listing and registration of therapeutic goods in
Australia, which helps to ensure the quality and safety of products. The processes vary
slightly for listed medicines, and low and high risk registered medicines; however, the
fundamental principles involved are similar.24 The process begins with the sponsor of a
medicine (the manufacturer or importer) submitting an application to TGA.24 For listed
medicines, the application must contain information relating to the quality and safety of the
product. The application is assessed by the ARTG section of the TGA to determine
whether ingredients in the product are suitable for listing. If the listing application is
successful, the sponsor will be issued with an AUST L number for the product.24
The process for registration of products is slightly more complicated, and a number of
different departments within TGA are involved. Sponsors who wish to register a non-
prescription (low-risk) medicine submit their application to the Chemicals and Non-
prescription Medicine branch of TGA.24 The application must include evidence of the
quality, safety and efficacy of the product. Depending on whether the application is for a
conventional non-prescription medicine or a complementary medicine, the application is
forwarded to the OTC Medicines Evaluation Section or the Office of Complementary
Medicines of TGA.24 If the application is for a new product, it will also be reviewed by a
relevant expert committee (the TGA Medicines Evaluation Committee or the
Complementary Medicines Evaluation Committee). A report is then given to TGA which
8
reviews the application and expert committee evaluation, and makes a final decision on
whether to approve the registration application.24
Applications for the registration of prescription (high-risk) medicines include detailed
information on the chemistry, safety, clinical effects and indication(s), adverse effects, and
pharmacokinetics of the medicine.26 These applications are submitted to the Drug Safety
and Evaluation Branch of TGA, which reviews the application and seeks advice from
external expert committees if necessary.24 Recommendations from these committees are
then submitted to the Australian Drug Evaluation Committee (ADEC), which provides a
final review of the application and makes a recommendation regarding whether the
application should be approved or rejected. ADEC membership includes medical
practitioners, pharmacologists, toxicologists and a manufacturing pharmaceutical
chemist.26 The Drug Safety Evaluation Branch of TGA considers the recommendation
from ADEC and makes the final decision on whether the medicine should be given
registration approval.24 This formal process helps to ensure the quality and safety of all
therapeutic goods available in Australia.
Safety of medicines following marketing approval in Australia is monitored by the
Adverse Drug Reactions Advisory Committee (ADRAC), a sub-committee of ADEC.18,26
Health care professionals and members of the public may report suspected adverse drug
reactions to ADRAC and drug companies are required by law to provide this information.
ADRAC maintains a register of these reports and produces a summary of results in a bi-
monthly bulletin for health professionals.26,27 If new information becomes available after
the listing or registration of a medicine such that TGA has serious concerns regarding the
quality or safety of the medicine, then licensing or regulatory approval may be withdrawn.
In addition to the process for registration of medicines, the Therapeutic Goods Act 1989
requires pharmaceutical manufacturers to be licensed to produce medicines for human
use.24 Licensed manufacturers must adhere to the “Australian Code of Good
Manufacturing Practice for Medicinal Products” which outlines the manufacturing
requirements to ensure the quality and safety of medicines manufactured in Australia.24,28
The aim of licensing of manufacturers is to ensure that medicines manufactured in
Australia meet high and specified quality standards.24 Manufacturers are audited by TGA
to ensure that their premises and manufacturing processes meet these standards.
9
2.1.3 Quality use of medicines
The third arm of Australia’s National Medicines Policy aims to ensure the quality use of
medicines. The conceptual framework used to achieve this is described in detail in the
National Strategy for Quality Use of Medicines.19 The overall goal of the National Strategy
for Quality Use of Medicines is “to optimise the use of medicines to improve health
outcomes for all Australians.”19 Quality use of medicines (QUM) is defined as:
−
“Judicious selection of management options. This means consideration of
the place of medicines in treating illness and maintaining health,
recognising that for the management of many disorders non-drug therapies
may be the best option;
−
Appropriate choice of medicines, where a medicine is considered
necessary. This means that, when medicines are required, selecting the best
option from the range available taking into account the individual, the
clinical condition, risks, benefits, dosage, length of treatment, co-
morbidities, other therapies and monitoring considerations. Appropriate
selection also requires a consideration of costs, both human and economic.
These costs should be considered both for the individual, the community
and the health system as a whole;
and
−
Safe and effective use. This means ensuring best possible outcomes of
therapy by monitoring outcomes, minimising misuse, over-use and under-
use, as well as improving the ability of all individuals to take appropriate
actions to solve medication-related problems, eg, adverse effects and
managing multiple medications.”19
One of the central principles involved in achieving QUM is partnership between all of the
stakeholders, who include consumers; health care providers, practitioners and educators;
the pharmaceutical industry; media and the government.19 In addition, the National
Strategy for QUM outlines six building blocks which have been shown to be integral in
supporting and achieving QUM:
•
“policy development and implementation;
•
facilitation and coordination of QUM initiatives;
•
provision of objective information and assurance of ethical promotion of medicines;
•
education and training;
•
provision of services and appropriate interventions; and
•
strategic research, evaluation and routine data collection.”19
The Pharmaceutical Health and Rational Use of Medicines (PHARM) committee is
responsible for overseeing the implementation and evaluation of the National Strategy for
Quality Use of Medicines.19 PHARM is an expert advisory committee with members
10
drawn from fields including medical practitioners, pharmacists, the pharmaceutical
industry, consumers, health educators and behavioural science.19
The National Prescribing Service (NPS) is a government funded, independent, non-profit
organisation which supports QUM by providing prescribing advice and implementing
programmes and guidelines to support rational and quality prescribing.29 A number of
QUM interventions are implemented by NPS, including academic detailing, prescriber self
audits, provision of case studies and continuing education meetings.29 The topics covered
in the interventions are chosen in response to criteria including General Practitioner (GP)
requests for education, where variability in the quality of prescribing in a certain area has
been identified, or when new prescribing guidelines are implemented.29 In addition to
providing services to prescribers, NPS also offers services for other health professionals
and for consumers.
2.1.4 A responsible and viable medicines industry in Australia
The fourth objective of the National Medicines Policy, maintaining a responsible and
viable medicines industry in Australia, began with the Factor f scheme in 1988.1 During
the 1980s activity of the pharmaceutical industry in Australia was declining. One of the
main factors noted to contribute to this decline was the low prices paid to pharmaceutical
companies for many PBS subsidised medicines compared to the prices paid for the same
medicines elsewhere in the world.1 There was a perception amongst the pharmaceutical
industry that Australia was a “hostile environment” for pharmaceutical manufacturing and
investment.1 The Factor f scheme was introduced in 1988 to try and reverse the trend of
declining pharmaceutical manufacture, research and development in Australia.1
Under the Factor f scheme, participating companies were able to receive higher prices for
some of their PBS listed medicines.1 In return, these companies had to increase their rate of
pharmaceutical export from Australia and increase their investment in pharmaceutical
research and development in Australia.1 Two phases of the scheme operated: Phase I
operated from 1988 to 1995; phase II overlapped with phase I, beginning in 1992 and
ending in 1999.1 Participation in phase I was based on a company’s ability to meet
eligibility criteria, including increasing pharmaceutical export and research and
development in Australia to levels predetermined by the government.1 All of the
companies who met these criteria were eligible to participate in phase I of the scheme.1
Similar criteria were used to determine eligibility for participation in phase II of the Factor
f scheme; however, companies also had to submit an application regarding their proposed
11
activity to increase pharmaceutical manufacturing and research and development in
Australia.1 Funding for phase II of the scheme was capped at $820 million and as a result a
number of pharmaceutical companies who met the eligibility criteria were unable to
participate, because the available funds had already been allocated.1
The Pharmaceutical Industry Investment Program (PIIP) replaced the Factor f scheme in
1999.30 Like the Factor f scheme, under PIIP pharmaceutical companies were able to
receive higher prices for some of their PBS subsidised medicines if they could prove that
the prices paid for these medicines under the PBS were lower than the prices paid for the
same medicines in the European Union.30 In return, these companies were required to
increase their pharmaceutical manufacturing and research and development in Australia.
Unlike the Factor f scheme, participation in PIIP was competitive. Pharmaceutical
companies were required to submit an application outlining their proposed increases to
pharmaceutical manufacturing and research and development activity in Australia.30
Acceptance into PIIP was based on the merit of the application.30
PIIP ended in 2004 and was replaced by the Pharmaceuticals Partnerships Program (P3),
which is scheduled to run until 2009.31 The aim of P3 is to increase the level of
pharmaceutical research and development in Australia, while at the same time promoting
partnerships between Australian and international pharmaceutical companies.32 Unlike
PIIP and the Factor f scheme, P3 doesn’t compensate pharmaceutical companies for low
prices paid for PBS medicines. Rather, it provides funding for pharmaceutical companies
who commit to increased pharmaceutical research and development in Australia.31
Participation in P3 is competitive and follows a similar process to participation in PIIP.
Initially, participating companies received reimbursement for 30% of the additional
amount spent on pharmaceutical research and development, up to a maximum of $10
million per company.31 This rebate has subsequently been increased to 50%.31
Evaluation of the Factor f scheme and PIIP showed that pharmaceutical activity in
Australia increased following introduction of the programs;1,30 although both evaluations
noted the difficulty of determining whether this increase in activity was a direct result of
Factor f and PIIP or whether it would have occurred anyway. The effect of P3 on helping to
maintain a viable pharmaceutical industry in Australia is yet to be evaluated.
12
2.2 Challenges for the supply and subsidy of medicines locally
and internationally
As discussed in the previous sections, one of the four central objectives of Australia’s
National Medicines Policy is “timely access to the medicines Australians need, at a cost
individuals and the community can afford”.14 The cost of subsidy of medicines on the PBS
and RPBS is largely borne by the community18 and reached $6.1 billion in 2006.3 The
governments of many other developed countries also subsidise the cost of medicines for
their residents and, similar to Australia, the costs associated with this subsidy are large.33
Amongst Organisation for Economic Co-operation and Development (OECD) countries in
2003, government subsidy of medicines accounted for 17.5% of total health care budgets
on average.34 A concern for Australia and other countries which subsidise medicines for
residents is the rising cost of pharmaceutical subsidy,35-40 which for many countries rose at
the rate of 10 to 12% each year in the late 1990s and early 2000s.41
The four objectives of Australia’s National Medicines Policy are interdependent, so
maintaining access to government subsidised medicines is essential to ensure that each of
the other National Medicines Policy arms, and the overall objective, are achieved. In an
attempt to achieve balance in the subsidy of medicines, while at the same time achieving
rational and effective use of medicines, policies targeting the market forces of supply and
demand have been implemented in the PBS and RPBS by the Australian government.
Similar policies have been implemented by other governments internationally.35,36 In the
following sections of this thesis, an overview of the economic theory of supply and
demand is provided. This forms a basis for the discussion of policies which are aimed at
influencing supply and demand of pharmaceutical products.
2.2.1 Supply and demand economic theory – an overview
In economic terms, a market is a means through which goods and services can be traded
between consumers and producers (or sellers).42,43 Markets operate with a demand side and
a supply side, where consumers tend to drive demand and producers drive supply.42-44 The
price of products available in a market is influenced by the number of producers of that
product and the prices for which they are willing to sell their product (supply); and by the
number of consumers for that product and the price the consumers are willing to pay
(demand).43 This is the basis for the economic theory of supply and demand.
Economic demand can be defined as “the relationship between the price of a good and the
quantity demanded”.43 The concept of demand requires that a consumer wants a product,
13
has the ability to pay for that product and is willing to pay the price sought for that
product.43 Supply of goods and services is influenced by a number of factors, notably the
price of producing that product and the price at which suppliers are willing to sell it.45
Economic supply can be defined as “the quantity of a good or service that sellers are
willing and able to sell at every conceivable price”.45
In perfect (or free) market situations, supply and demand adjusts in response to “market
signals”, which in simple terms are related to the price and quantity of goods sold.42 An
example of a market signal is when a consumer desires a product, but cannot afford or does
not wish to pay the sale price and so does not purchase the product. In response to this
market signal, the producer reduces the sale price and in response to the market signal of
reduced price the consumer purchases the product.42 A number of assumptions are made
about consumers and producers in order for this to occur, which are assumed to hold true
in the perfect market place.42
An assumption made about consumers is that they make rational decisions about the goods
they purchase.42-44 It is assumed that in making the decision to purchase a product,
consumers weigh up the potential benefits of the product with the associated cost.42-44
However, in the case of the use of healthcare and medicines, this is often not the case.
Consumers frequently rely on the advice of health care professionals to determine
appropriate healthcare treatments, and in many cases these treatments (such as specialist
referrals or the use of prescription medicines) can only occur with health care professional
input. In this situation, health care professionals influence demand.43
An assumption made about producers is that they are price competitive.42 In the perfect
market place, it is assumed that there are many producers of small size, so that a single
producer cannot exert a monopoly over the market.42 In this situation, in order to maximise
their profits, producers must manufacture their product cost effectively, to in turn offer the
product for sale at a low price attractive to consumers.42,44 If producers are not cost
effective in manufacturing, then their sale price will be higher and consumers will choose
to purchase that product from a cheaper producer.42 These assumptions about the
behaviour of consumers and producers allow predictions to be made regarding the changes
that will occur in demand or supply in response to changes in the price of a product,42,44 as
depicted in Figure 2.2.
As depicted on the demand curve in Figure 2.2, a decrease in the price of a product (shown
on the vertical axis) may lead to an increase in the quantity demanded (depicted on the
14
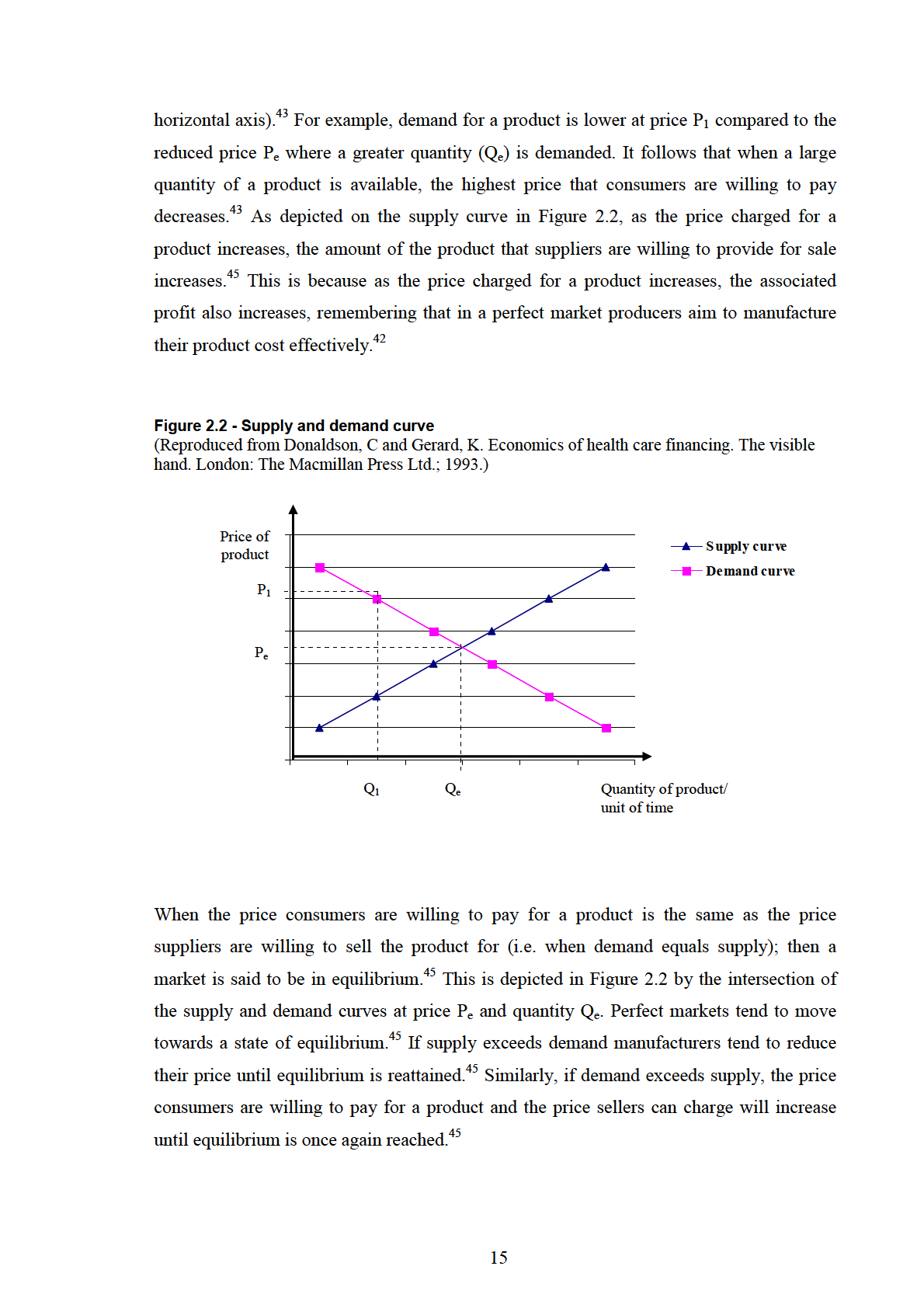
The supply and demand theory discussed in the previous section operates in a perfect
market place. However, many of the assumptions central to the theory do not apply in the
pharmaceutical market. In a perfect market, it is assumed that consumers are fully
informed and able to make rational decisions about purchases; however this is frequently
not the case when it comes to the purchase of prescription medicines.42-44 Doctors and
pharmacists are necessarily involved in the process as the prescribers and dispensers of
medicine. Another assumption made is that the market consists of a number of small
producers.42 In the pharmaceutical market, availability of medicines depends on a number
of external factors in addition to availability of the product – for example most countries,
including Australia, require a medicine to be registered before it can be used. The number
of producers of a medicine may be regulated or influenced by government policies, so the
assumption that a market consists of numerous small producers may not hold in the
pharmaceutical market.42 Medicines which receive a government or health insurance
subsidy are cheaper to consumers and are therefore a more attractive option for consumers
than non-subsidised medicines. The assumption that suppliers aim to maximise their profit
does not hold, because in many countries governments are involved in the purchase of
medicines and influence the prices paid for medicines.45 No markets function in perfect
conditions,42 and these examples highlight some of the areas in the pharmaceutical market
where assumptions made in supply and demand theory do not hold.
Many governments in developed countries have introduced policies aimed at influencing
the supply and demand of pharmaceutical products, in an attempt to achieve a balance
between the prices paid for medicines and the quantity of medicines consumed without
adversely impacting on the safe or rational use of medicines. Although the systems for
pharmaceutical supply and subsidy are different between many countries, the policies used
in an attempt to control pharmaceutical supply and demand tend to be similar.38 These
policies are generally targeted at stakeholders in the pharmaceutical market, in particular
patients, healthcare providers and the pharmaceutical industry.35,38
2.2.2 Pharmaceutical policies to influence demand
Pharmaceutical policies targeted to influence consumer and practitioner demand
commonly regulate the price of, or access to, medicines. These policies include
prescription cost-sharing, listing systems or formularies, prescribing guidelines or
restrictions and physician budgetary restrictions; and are described in the following
sections of this chapter.
16
2.2.2(i) Prescription cost-sharing
A strategy to control demand for pharmaceutical products is cost sharing for prescription
medicines. The use of medicines is thought to be “price sensitive”, meaning that use of
medicines is likely to be low when costs are high and, conversely, use is likely to be high
when costs are low.39 Prescription cost-sharing requires patients to contribute a proportion
of the cost of their medicines. The rationale behind cost sharing is to minimise the
unnecessary over-use of pharmaceuticals that may occur when costs are low.35,38,39,46
Different countries and health insurers use different systems of prescription cost-sharing.
In Australia,39 Germany38 and Britain35 fixed patient co-payments apply. Many American
health insurance organisations have tiered co-payments, where patients pay a lower fixed
co-payment for “preferred” medicines and generic medicines, and a higher fixed co-
payment for “non-preferred” medicines and brand name medicines.47 In some European
countries, including Switzerland, the Netherlands, Spain and Norway, patients contribute a
set percentage towards the cost of their medicines.38 In other European countries, including
Finland and Italy, patients also contribute a proportion of the actual cost of their medicines;
however, the proportion contributed varies depending on the medicine used.38 Despite the
differences in the type of cost-sharing strategies between countries, the intention of cost
sharing policies tends to be the same – that is, to provide patients with a financial incentive
to rationalise their use of medicines, without leading to worsened health outcomes as a
result.35,38,39
2.2.2(ii) Formularies
Another common method used to influence pharmaceutical demand is to limit the
medicines that are available for reimbursement.38 This may take the form of a “positive
list” or formulary, where only the medicines which are included on the list are available for
subsidy; or a “negative list”, where medicines on the list are excluded from subsidy.38,46
The price of medicines on the formulary is usually less than the price of un-listed
medicines, thereby creating an incentive (or demand) for patients to consume medicines on
the formulary and for physicians to prescribe these medicines.
Generally, medicines needed for the management of serious or chronic conditions, which
have proven efficacy, are included on positive lists.38 Medicines with un-proven efficacy or
those used for the treatment of non-serious conditions tend to be excluded from positive
lists, and tend to be those included on negative lists.38 Countries with positive lists (or
formularies) include Australia and Switzerland, while in England a negative list of over
17
3000 medicines which are excluded from government subsidy applies.38 Most health
insurance organisations in the USA also use formularies.48
2.2.2(iii) Generic substitution policies
Many countries have policies to encourage the use of generic medicines.38 Generic
medicines are usually cheaper than brand name medicines. If patients are required to pay
the actual price of medicines, or are required to pay the extra cost for brand name
medicines compared to generic medicines, then demand for generic medicines should
increase. A number of European countries, including Denmark, Germany, the Netherlands
and Sweden have generic substitution policies in place,38 as does Australia.2 These policies
facilitate the use of generic medicines by allowing patients to receive generic products
even if they have been prescribed a brand name product.38 Generic substitution policies
facilitate increased demand for cheaper generic medicines.
2.2.2(iv) Prescribing feedback, education and guidelines
In an attempt to inform doctors about the cost of the medicines they prescribe, many
countries have prescriber feedback systems.49 The rationale behind prescriber feedback is
that if doctors are aware that their prescribing costs are higher than their colleagues, they
may rationalise their prescribing and reduce costs.49 In Britain information is provided to
general practitioners (GPs) regarding the overall cost of medicines prescribed in their
practice and a comparison with the prescribing costs of other practices in the area is
made.49 In Germany, doctors are provided with a summary of their prescribing costs and a
comparison is also made to the prescribing costs of their colleagues.49 Feedback systems
like these have a limited impact on prescribing because participation is usually voluntary
and the information provided may be ignored.49 In addition, doctors are not provided with
education or strategies to improve their prescribing in areas where costs may be
unnecessarily high or where prescribing is inappropriate.
In Australia, the need for prescriber feedback and education is highlighted in the quality
use of medicines (QUM) arm of the National Medicines Policy.19 One of the six building
blocks outlined in the National Strategy for Quality Use of Medicines, identified as being
necessary to achieve QUM, is “access to education and training to support best practice”.19
The National Prescribing Service (NPS) plays a large role in the delivery of prescriber
feedback and education in Australia. The prescriber feedback programmes incorporate
education and information with feedback on prescribing patterns.29 This strategy enables
doctors to “self-direct” prescribing changes.29 Similar programs operate in New Zealand40
18
and evaluation of programs in both countries has shown an improvement in doctors
prescribing patterns following educational interventions and feedback.29,40
2.2.2(v) Prescribing budget restrictions
Some countries have introduced drug budgets in an attempt to control the costs of
subsidising medicines.38,49 Drug budgets can be applied to individuals or regions. For
example, throughout the 1990s prescribing budgets for individual practices were used in
Britain49 and regional prescribing budgets were introduced in Germany.38 If prescription
drug spending for the region exceeded the budget, then physician organisations for the area
were required to pay the price difference.38,49 Prescribing budgets aim to reduce demand
for pharmaceutical products by providing a financial incentive for doctors to prescribe
medicines rationally.38 Following the logic of demand theory, if prescribers exceed their
drug budget, then the cost to the doctor will increase. This potential increase in costs will
theoretically lead to a decrease in demand for pharmaceutical products.
2.2.3 Pharmaceutical policies to influence supply
The most common government policies designed to influence supply of medicines focus
on the prices the government is willing to pay for medicines. Because many governments
around the world subsidise the cost of medicines for consumers, policies controlling the
amount a government pays the manufacturers of these medicines will influence supply.
Governments may also influence supply of medicines by licensing and regulatory
requirements. Policies designed to influence the supply of medicines include regulatory
approval, reference pricing policies and cost effectiveness analysis, and are discussed in
the following sections.
2.2.3(i) Medicines licensing and regulatory approval
Nearly all countries require medicines to be licensed or registered before they can be sold
or used in that country.38,46,50 Licensing involves an assessment of the quality, safety and
efficacy of products. Licensing and regulation requirements are one of the most effective
ways of controlling supply of medicines in a market, because unlicensed products are
prohibited from sale.46 This provides an incentive for manufacturers to conduct the studies
required to prove the safety and efficacy of their products prior to market.
2.2.3(ii) Reference pricing policies
Reference pricing policies are used by many governments in an attempt to regulate the
prices paid for medicines. Reference pricing schemes apply to medicines within the same
therapeutic class which are considered to be therapeutically interchangeable.36,38,50 A
19
reference price is set for the medicines in that therapeutic category and represents the
maximum reimbursement price.36,38,50 Patients who use more expensive medicines in the
therapeutic group are required to pay the difference between the actual cost of the medicine
and the reference price.36,38,50 The method used to determine the reference price varies
between countries. Commonly, it is the price of the lowest cost medicine(s) within a
therapeutic category, as is the case in New Zealand50 and Denmark.36 In Sweden, the
reference price is determined by the price of the lowest cost generic medicine within the
therapeutic category plus a set additional amount, currently 10%.36,50 In the Netherlands
and Germany, the average price of medicines within the therapeutic group is used as the
reference price.36,50 The economic theory behind reference pricing is that there is an
incentive for manufacturers of more expensive medicines to reduce their prices to, or close
to, the reference price.38,50 If they don’t, they run the risk of losing market share if patients
switch from more expensive products to the reference priced product.36
2.2.3(iii) Cost effectiveness pricing policies and economic evaluation
A number of governments attempt to control supply of medicines by cost effectiveness
evaluation.36,50 Cost effectiveness evaluation requires a pharmaceutical company to
provide evidence of the benefits of their medicine relative to other available treatments, to
justify the price requested for that medicine.36,50 Drug manufacturers who cannot show that
their medicine is cost effective compared to other available treatments may not have their
medicine subsidised by the government or health insurer, or may not receive the price
requested for that medicine. Countries including Australia, Finland, France, Portugal,
Sweden, and Ireland, and some provinces of Canada use cost effectiveness analyses to help
regulate the supply of medicines.36,50 Suppliers of medicine have a strong incentive to
prove the cost effectiveness of their product, or to reduce the price requested for their
product to the price of other medicines of similar safety and efficacy. If they don’t do this,
they run the risk of not receiving government subsidy or formulary listing for their
medicine.
2.3 Policies influencing supply and demand, implemented to
ensure continued access to medicines in Australia via the PBS
and RPBS
In the previous sections of this chapter, the types of policies used around the world in an
attempt to regulate the supply and demand of medicines were described. A number of
policies have been introduced to the PBS and RPBS which aim to influence supply and
demand of medicines and thereby help control the costs of government subsidy of
20
medicines. These policies were introduced to the PBS and RPBS in response to
government concerns about the rising cost of medicine subsidy, which has increased most
years since introduction of the PBS (Figure 2.3). In 1980, PBS medicines subsidy cost the
government $275 million, and by 1990 this had increased to over $1 billion.17 The annual
government expenditure on the PBS increased by around 10% each year between June
1999 and 2002, however the number of prescriptions dispensed each year did not increase
at the same rate.51 In recent years the cost has still increased, although to a lesser extent.
The government cost of the PBS was $6,001.2 million in the 2004-05 financial year52 and
increased to $6,163.1 million in the 2005-06 financial year.53
Figure 2.3 - Increasing Government cost of PBS subsidy
Financial year
Government costs
(July 1st – June 30th)
($ million)
1959 - 1960
4917
1969 - 1970
13717
1979 - 1980
27517
1989 - 1990
1,17917
1999 - 2000
3,187.254
2005 - 2006
6,163.13
Strategies used to influence supply and demand of PBS medicines include formal
processes for the listing and pricing of PBS medicines, which have been in place since
soon after PBS implementation.17 Initially, PBS medicines were available free of charge,
however, the introduction of patient co-payments in 1960 and subsequent increases over
ensuing years has transferred some of the cost of the PBS and RPBS from the government
to consumers.17 Cost effectiveness assessments were introduced for PBS listing of
medicines in 1987 and were made compulsory in 1993.55 In 1990 a pricing policy for
medicines with multiple brand and generic products, the minimum pricing policy, was
introduced; followed by a brand substitution policy in 1994 and a reference pricing policy
known as the therapeutic group premium policy in 1998.56,57 These PBS policies designed
to influence supply and demand of medicines are discussed in detail in the following
sections of this chapter.
21
2.3.1 Pricing and listing of PBS medicines
In order for a medicine to be considered for PBS listing the manufacturer must make a
submission to the Pharmaceutical Benefits Advisory Committee (PBAC). PBAC assesses
medicines for listing on the PBS and advises the Government Minister for Health and
Ageing accordingly.58 Doctors, pharmacologists, pharmacists, consumers and health
economists are represented in membership on the PBAC.58 The National Health Act 1953
requires PBAC to take into account both cost, and comparative efficacy and safety of
medicines when making PBS listing recommendations, and manufacturers are required to
submit a cost effectiveness analysis when applying to the PBAC for PBS listing of a
medicine.41,59
If PBAC makes a positive recommendation for PBS listing, the Government Minister for
Health and Ageing may then accept or reject the recommendation. Following acceptance
of a recommendation, the Pharmaceutical Benefits Pricing Authority (PBPA) will
recommend the price the government should pay for a medicine.59 A number of factors are
taken into account by PBPA when making price recommendations. These include the
recommendations of PBAC, the cost of other medicines in the same therapeutic class that
are already listed on the PBS and prices paid for the medicine in other countries (in
particular New Zealand and the United Kingdom).59 If pharmaceutical manufacturers do
not agree to pricing recommendations made by PBPA then the medicine may not be listed
on the PBS. Alternatively, a “special patient contribution” may apply. In this situation the
difference between the price the Australian Government is willing to pay for the medicine
and the price the pharmaceutical company wishes to charge for the medicine is payable by
patients in addition to the standard co-payment. The extra charge is referred to as a special
patient contribution and is a manufacturer charge.3 Special patient contributions apply
rarely and in 2006, only six of the more than 600 medicines available on the PBS were
subject to the extra charge.3
2.3.2 Patient co-payments
Patient co-payments were introduced to the PBS and RPBS in an attempt to influence
patient demand. When the PBS was first implemented all listed medicines were available
to patients free of charge.17,20,27 In 1960 a co-payment equivalent to 50 cents was
introduced for non-pensioners and by 1983 it had risen to $4.00 per PBS prescription.17 In
response to concerns that this cost was prohibitive for patients of low income the
concession category with a co-payment of $2.00 per PBS prescription was introduced in
1983 (Figure 2.4).17 Co-payments for aged pensioners were introduced in 1990 and for
22
DVA treatment card holders in 1992.17 Since 1992 co-payments for people of low income,
aged pensioners and DVA card holders have been the same (the concession category).17
The last major increase to patient co-payments was seen in 2005, when the general co-
payment increased by nearly $5.00 and the concession co-payment increased by 80c
(Figure 2.4). In 2008, co-payments were set at the level of $31.30 per prescription for
general patients and $5.00 per prescription for concession patients.23
Figure 2.4 - Major PBS and RPBS co-payment changes
March 1960
General co-payment of 50c introduced
1971 –1981
General co-payment increased from $1.00 to $3.20 in small (50c or
less) increments
January 1983
General co-payment increased to $4.00
Concession co-payment of $2.00 introduced
July 1985
General co-payment increased to $5.00
November 1986 General co-payment increased to $10.00
Concession co-payment increased to $2.50
November 1990 General co-payment increased to $15.00
Concession co-payment extended to include aged pensioners
January 1992
Co-payment of $2.50 introduced to RPBS
1992 - 2004
Small yearly co-payment increases, less than $1.00 per year for
general co-payments and around 10c per year for concession patients
January 2005
General co-payment increased by $4.90 (from $23.70 to $28.60)
Concession co-payment increased by 80c (from $3.80 to $4.60)
2.3.2(i) Safety Net provisions
Safety Net provisions were introduced to the PBS and RPBS following the large co-
payment increases in 1986 (Figure 2.4). The government of the time recognized that such a
large increase could shift the burden of cost to patients; particularly to ill patients who
required many PBS medicines.17 Under Safety Net provisions, patients pay co-payments
for their medicines until they reach a “threshold”, after which time all remaining PBS
prescriptions are free (for concession patients) or available at the concession co-payment
(for general patients) for the remainder of the calendar year.17 The level of the Safety Net
threshold has varied since implementation; however, it is currently based on prescription
expenditure. In 2008, general patients were required to spend $1141.80 per family
(equivalent to 37 general PBS co-payments) before reaching the Safety Net.23 The
23
threshold for concession patients in 2008 was $290.00 per family, equivalent to 58
concessional PBS co-payments.23
2.3.3 The minimum pricing policy
The first pricing policy for different brand and generic products of the same medicine, the
generic pricing policy, was introduced to the PBS in 1983. Under the policy, manufacturers
of more expensive products were required to reduce their prices to a set maximum above
the price of the cheapest generic in order to maintain their PBS listing. Initially this price
differential was only 5c.17 The generic pricing policy was unpopular amongst drug
companies, as the government effectively forced them to reduce their prices or have their
medicine de-listed from the PBS.17 The policy was ultimately unworkable, with numerous
instances where medicines were de-listed from the PBS only to be subsequently re-listed at
a later date.17
The minimum pricing policy was introduced to the PBS in 1990 to replace the generic
pricing policy. It represented a policy change allowing manufacturers of medicines with
generics available to set the price for their product, and was designed to influence patient
demand for medicines.1 The minimum pricing policy applies when there is more than one
brand or generic product available for a medicine. PBS and RPBS medicines are subsidised
up to the price of the lowest priced product(s) of that medicine,1 commonly referred to as
the benchmark priced product(s). Patients who use benchmark priced products pay only the
patient co-payment. Those who use more expensive brands are required to pay the price
difference between the benchmark priced and more expensive product, in addition to the
co-payment.1 This extra charge is referred to as a brand premium.1 The minimum pricing
policy provides a price signal to patients regarding the cost of different products of the
same medicine.1 Manufacturers who set their prices too high above the benchmark priced
product run the risk of patients switching to cheaper products to avoid paying large brand
premiums. In 2006, the price difference between benchmark and premium priced products
was between $1 and $4 for most PBS medicines.3 At least one product for each strength of
medicine on the PBS is available to patients without attracting a brand premium.59
2.3.3(i) Brand substitution by pharmacists
When the minimum pricing policy was introduced, pharmacists were required to dispense
the brand of medicine prescribed by the doctor. Patients prescribed more expensive
products were required to pay brand premiums in addition to the PBS co-payment, or liaise
with their doctor to have their prescription written for a benchmark priced product. The
24
National Health Act was amended in 1994 to allow brand substitution by pharmacists.1
Brand substitution can occur at the time of dispensing without prescriber consultation, if
the patient agrees to the product change, if the substituted products are indicated as being
bioequivalent in the Schedule of Pharmaceutical Benefits and if the prescriber has not
specified that substitution cannot occur.2 Prescribers may prohibit substitution by marking
the prescription “brand substitution not permitted”.2
The brand substitution policy facilitates use of cheaper products, by giving patients the
opportunity to respond to the price signal associated with more expensive brands of
medicine. Rather than having to consult their doctor for a prescription for a cheaper brand
of medicine, patients can choose to receive a benchmark priced product at the time of
dispensing if they don’t wish to pay a brand premium.
The terms “brand substitution” and “generic substitution” are used interchangeably in the
Australian setting when describing the substitution of pharmaceutical products. Generic
medicines are marketed using a unique trade name rather than just the generic name of the
medicine and different generic products are often referred to as generic “brands”. In this
context, use of both terms to describe interchange of brand or generic products is
appropriate. However, throughout this thesis the term “brand substitution” will be used to
describe substitution of different pharmaceutical products under the brand substitution
policy.
2.3.3(ii) Bioequivalence of brand and generic products
One of the criteria for brand substitution of PBS and RPBS medicines is that the
substituted products are bioequivalent. If a pharmaceutical company wishes to market a
new generic product in Australia which is interchangeable with products already available,
they must provide the Therapeutic Goods Administration (TGA) with evidence of
bioequivalence to the original brand name product.60 According to the definition used by
TGA, generic products are bioequivalent to the original brand if they contain the same
amount of the same active ingredient in an identical dosage form; and if the active
ingredient is absorbed in the body to reach the site of action at the same rate and to the
same extent as the original product.61
Manufacturers must provide TGA with evidence of bioequivalence between a new generic
and the original brand name product from a bioequivalence study.61 Bioequivalence studies
are conducted under standardized conditions using a minimum of twelve volunteers.61
Bioequivalence is assessed using the area under the curve (AUC) ratio and maximum
25
plasma concentration (Cmax) ratio of the generic and original brand name products. AUC is
a measure of the extent of absorption of the medicine while Cmax provides a measure of the
rate of absorption of the product.62 Under TGA regulations, a generic product is
bioequivalent to the innovator product if the 90% confidence intervals for the AUC ratio
and Cmax ratio lie between 80% and 125%.62
2.3.4 The therapeutic group premium policy
Another major PBS policy change implemented in 1998 was the therapeutic group
premium policy, a form of reference pricing.2 It is based on similar principles to the
minimum pricing policy, however, it applies to medicines within a therapeutic class rather
than different brand and generic products of the same medicine. When PBAC recommends
that medicines within a therapeutic group are of such similar safety and efficacy that they
can be interchanged without any expected change in therapeutic outcomes, the therapeutic
group premium policy may apply.59,63 The lowest priced medicine within each therapeutic
group sets the “benchmark” price for other medicines.59 Manufacturers of other medicines
within the group must reduce their price to the benchmark price or a therapeutic group
premium will apply. This is the price difference between the benchmark priced and more
expensive medicine. Patients must pay this extra charge in addition to the co-payment.
Manufacturers who don’t reduce their price to the benchmark price run the risk of patients
responding to the price signal of the therapeutic group premium and requesting a
prescription for a cheaper medicine from their doctor. Unlike the minimum pricing and
brand substitution policies, pharmacists cannot substitute between different medicines
under the therapeutic group premium policy.59
The therapeutic group premium policy currently applies to five groups of medicines: the
histamine-2 (H2) receptor antagonists, dihydropyridine calcium channel blockers,
angiotensin converting enzyme (ACE) inhibitors, and statins.59 Currently, none of the
statins attracts a therapeutic group premium because all manufacturers reduced their prices
to the benchmark price.64 If prescribers think that patients may become confused by
switching to a benchmark priced medicine or if drug interactions will occur or are expected
to occur by switching medicines, the prescriber can apply for exemptions to therapeutic
group premiums for their patient. This takes the form of an “authority” prescription.59
26
2.4 Co-payments, the minimum pricing policy and therapeutic
group premium policy: influencing supply and demand on the
PBS and RPBS
Introduction of patient co-payments, the minimum pricing policy, the brand substitution
policy and the therapeutic group premium policy were designed to influence demand for
PBS and RPBS medicines. As discussed in section 2.2, when a product is available for low
cost then consumer demand for that product will be high. Co-payments increase the cost of
medicines to patients, meaning that demand for medicines should decrease. Similarly, the
minimum pricing policy means that some brands of medicine are more expensive than
others and the therapeutic group premium policy means that some medicines within a
therapeutic group are more expensive than others. The economic theory described in
section 2.2 suggests that this should send a price signal to patients, and demand for co-
payment priced products should increase while demand for the more expensive medicines
should decrease. At the same time, this also sends a market signal to manufacturers that
they run the risk of losing market share if they do not reduce the price of their medicine to
(or near to) the benchmark price.
Although these policies are designed to influence the prices paid for medicines in Australia
and the volume of government subsidised medicines consumed by Australian residents, as
described in section 2.2 many of the assumptions made in supply and demand theory do
not hold in the pharmaceutical market place.42-44 Implementation of policies designed to
influence supply and demand of medicines may therefore have unintended effects. In the
following chapter, the literature surrounding the impact of introduction of patient co-
payments, the minimum pricing and brand substitution policies and the therapeutic group
premium policy on the use of PBS medicines is reviewed.
27
CHAPTER 3
PBS policies to influence supply and demand: what is the
impact on achieving the National Medicines Policy
objectives?
Policies implemented within Australia’s National Medicines Policy should help to achieve
the overall aims of the National Medicines Policy.19 Analysis of the impact of these
policies on achieving the National Medicines Policy aims is important.18 As discussed in
Chapter 2, policies intended to influence pharmaceutical supply and demand, introduced to
the access arm of the National Medicines Policy via the PBS and RPBS, may have
unintended effects. This can occur because some of the assumptions made in supply and
demand theory do not hold true in pharmaceutical markets.42-44 There is the potential for
policies designed to influence supply and demand of medicines to impact on achieving
other objectives of the National Medicines Policy, in particular the quality use of
medicines. In this chapter research on the effect of increasing patient co-payments, the
introduction of the minimum pricing and brand substitution policies, and the introduction
of the therapeutic group premium policy on the use of medicines is reviewed. The
implications of this are discussed from the quality use of medicines perspective.
3.1 Review of the literature on PBS patient co-payments
When people have health insurance, they tend to be greater consumers of health services
than they would be if they were uninsured and had to pay the full cost of the health
service.65 This also applies when people are covered by schemes like the PBS and RPBS
which make the cost of medicines to the patient lower than it otherwise would be.65 The
term moral hazard is used to describe the scenario where medicine (or health resource) use
by consumers is increased, and perhaps unnecessarily, when the cost to the consumer is
low.39,65 Prescription co-payments are designed to minimise the unnecessary or excessive
use of medicines that could potentially occur when medicines are available for no charge
28
or for a very low price, and it was for this reason that co-payments were originally
introduced to the PBS and RPBS.39
Since the introduction of co-payments to the PBS and RPBS it has been suggested that
moral hazard still operates because Australians are so accustomed to the availability of
medicines at a relatively affordable price due to PBS and RPBS subsidy.39 In particular, the
safety net provisions on the PBS and RPBS have been shown to contribute to moral
hazard. Under safety net provisions, patients pay co-payments for medicines until they
reach a “threshold”, after which time all remaining PBS prescriptions are free (for
concession beneficiaries) or available at the concession co-payment (for general
beneficiaries) for the remainder of the calendar year.17 In the five years post introduction of
the safety net, there was a large increase in the number of PBS and RPBS medicines
dispensed in the months at the end of each calendar year, associated with increased
numbers of patients qualifying for the safety net and having multiple prescriptions
dispensed at the reduced price. This was followed by a decrease in the number of PBS and
RPBS medicines dispensed at the start of the following year as patients reverted from
safety net provisions to the usual PBS co-payment.66 The decline in PBS and RPBS
medicine dispensing seen at the start of each calendar year was attributed to patients
consuming the “stockpile” of medicines which had been dispensed to them in the latter
months of the prior calendar year.66
In response to the moral hazard and medicine stockpiling associated with introduction of
the safety net provisions, a minimum re-supply interval was introduced for many PBS and
RPBS medicines in November 1994.67 Prior to this, repeat PBS prescriptions could be
dispensed within three days. After the minimum re-supply interval was increased twenty
days had to elapse before a repeat dispensing was allowed.67 A study was conducted to
evaluate the effect of this policy change on medicine dispensings. Monthly dispensing
patterns between January 1991 and June 2000 were compared using PBS dispensing claims
data.67 Results of the study showed that, after the minimum re-supply interval was
increased, the peaks in the number of medicines dispensed in December each year were
lower.67 In the years before the change, around 14% of all PBS and RPBS prescriptions
dispensed each year were dispensed in December. In December 1994, just after the
minimum re-supply interval increased, only 10% of the yearly prescription total was
dispensed.67 This figure remained relatively stable over ensuing years, with 11% of all PBS
and RPBS prescriptions dispensed in December 1999.67 Although increasing the re-supply
interval to twenty days reduced moral hazard associated with decreased cost of medicines
29
after patients reached the safety net, a high proportion of medicines were still dispensed in
December each year. This suggests that there is still some degree of moral hazard
operating on the PBS.
Prescription co-payments are therefore necessary, because Australian research has clearly
shown that moral hazard operates when PBS and RPBS medicines are available to patients
free of charge after reaching the safety net. The stockpiling of medicines that occurs in the
latter months of each calendar year may result in poor quality use of medicines and patient
harm if patients continue to use “stockpiled” medicines after therapy has been changed by
the doctor (e.g. a change in medicine or dose) or if medicines expire. Patient co-payments
are designed to send a price signal to patients so that they only have medicines dispensed
when they are needed, and so that, in consultation with health professionals, they
rationalise their medicine use and do not use government subsidised medicines
unnecessarily.39 However, an unintended effect of increasing co-payments may be that
patients cannot afford to pay for the medicines they need to maintain their health, and
therefore do not have them dispensed.68-70
Quantitative Australian research studied changes in the number of prescription medicines
dispensed following introduction of the co-payment for aged pensioners and a $4 increase
to the general patient co-payment in November 1990.68 Changes to RPBS medicine
dispensing following introduction of the RPBS co-payment in 1992 were also studied.68
The number of PBS and RPBS prescriptions dispensed pre and post the co-payment
changes was compared using an interrupted time series design, using PBS administrative
claims data.68 Piecewise, segmented linear regression analysis was used to identify changes
in prescription levels after the co-payment changes. Medicines used for the treatment of
short term conditions or symptom relief were defined as discretionary; while medicines
necessary for the management or treatment of chronic conditions were defined as
essential.68
Following the co-payment changes the number of government subsidised prescriptions
dispensed decreased.68 Compared to the prescription levels prior to the co-payment
changes, dispensing of discretionary medicines on the PBS was lower post co-payment
changes, with a decrease in the level of the series of 758,500 prescriptions (95% CI
901,189 - 615,813; p < 0.001).68 Compared to the dispensing trend prior to the co-payment
changes, dispensing of discretionary medicines after the co-payment changes was 24.8%
lower than expected.68 Dispensing of prescriptions for discretionary medicines on the
30
RPBS also decreased after introduction of the co-payment, with a decrease in the level of
the series of 32,500 prescriptions (95% CI 44,442 – 20,510; p < 0.001).68
Dispensing of essential medicines also decreased after the co-payment changes. There was
a decrease in the level of essential medicines dispensed on the PBS by 816,000
prescriptions (95% CI 1,116,133 – 516,373; p < 0.001) and a decrease in the level of
essential medicines dispensed on the RPBS by 29,500 prescriptions (95% CI 45,812 –
13,287; p < 0.001). Dispensing of essential medicines after the co-payment changes was
18% lower than expected compared to the dispensing trend prior to the changes.68 Overall,
in the year post co-payment changes dispensing of government subsidised prescriptions
fell by 16% and associated costs fell by 22%; however, this decrease was not maintained in
following years.68
These results suggest that increases to patient co-payments result in rationalisation of
medicines use, because the number of prescriptions dispensed for discretionary medicines
decreased after the co-payment changes. However, it is of concern that the number of
prescriptions dispensed for essential medicines also decreased following the co-payment
changes. This study did not assess the impact of essential medicine cessation on patient
health outcomes. It is possible that cessation of essential medicines led to worsened health
outcomes for some patients, and that some of the cost savings associated with the co-
payment changes were offset by increased health resource utilisation by patients.
Patient surveys regarding co-payment increases have highlighted some of the issues
patients face in affording their medicines.69,70 In 1993, two years after the introduction of
co-payments for aged pensioners, a national telephone survey was conducted to determine
pensioner awareness of co-payments and the impact co-payments had on the affordability
and use of medicines.69 A representative sample of 433 pensioners from suburban and rural
areas of all five mainland Australian states participated. Ten percent of respondents
reported that since introduction of the co-payment they had gone without a prescription
medicine due to the purchase cost.69 Single parents and patients receiving a sickness or
disability pension were most likely to report this.69 When pensioner co-payments were
introduced, the fortnightly pension was increased by an amount equivalent to two PBS co-
payments to compensate for the co-payment cost;17 however, 33% of respondents reported
spending more than this amount on their prescription medicines.69 Forty percent of
pensioners surveyed thought that people using multiple medicines, people of lower income
and the chronically ill were most likely to be disadvantaged by introduction of co-
payments.69
31
The most recent analysis of the influence of PBS co-payments on the use of medicines was
conducted between 2000 and 2001, and similar concerns were highlighted.70 To identify
the extent to which co-payments influence the use of medicines in Australia, two surveys
and an interview study were conducted. The first survey, a consumer survey, was mailed to
1,000 residents in New South Wales. Survey questions aimed to identify difficulties
patients faced in affording medicines. Of the 420 respondents (response rate 44%), 45%
indicated that cost was an issue in obtaining medicines.70 Twenty percent of respondents
reported not being able to afford to have prescriptions dispensed.70 The consumer survey
also highlighted that quality use of medicines can potentially be compromised by
prescription costs. A quarter of respondents reported using a medicine that had been
prescribed for a previous illness due to the cost of having a new prescription dispensed,
while 13% admitted sharing medicines amongst family members and 10% reported using a
medicine prescribed for someone else due to cost.70 One in ten respondents also reported
taking less than the prescribed dose because of medicine costs.70 Respondents from
families with children were twice as likely to report problems affording medication than
others.70
Patients who had visited a GP at one of 20 surgeries in New South Wales were invited to
complete the second survey. Of the 1,036 eligible participants, 442 (43%) responded. The
second survey included questions designed to identify the difficulties patients face in
affording their medicines. Similar themes to the community survey emerged. Many
patients described cost as an issue that delayed them from seeking GP treatment.70 A
number of patients noted that a GP consultation was likely to result in a prescription and
that it was the cost of the prescription that delayed seeking GP treatment.70 A number of
patients who reported receiving a PBS prescription at their GP encounter did not have it
dispensed due to the cost.70 Socioeconomic status appeared to be associated with
difficulties in affording prescription medicines, with less patients in high income categories
compared to middle and low income earners reporting that they did not have a prescription
filled due to the cost.70
Thirty three consumers participated in the interview component of this study. They were
identified from the consumer survey and had indicated willingness to participate in an
interview on the topic of prescription co-payments.70 An emerging theme from the
interviews was that if patients had made the decision to spend their time and money
consulting a doctor there was “little sense” in not having a prescription dispensed.70 This
theme was reported amongst all patients, including those who also reported difficulties in
32
affording medicines.70 These findings are in contrast to the findings of the surveys, where
patients frequently described not having prescriptions dispensed. The patients interviewed
tended to feel that when their doctor wrote a prescription it was “an expression of expert
clinical judgement” and that if the doctor had prescribed it, then it was a necessary
therapy.70 This may explain the different results from the surveys and interviews. Patients
in the interviews may have been less willing to admit not having had a prescription
medicine dispensed due to the cost after acknowledging to the interviewer that, having
been prescribed, the medicines were necessary. However, it may also be a reflection of the
small interview sample. The patients who agreed to participate in the interviews may have
been motivated to do so because of their views towards medicines, and their views may not
be representative of the wider population. Participants in the study were drawn from a
single region in a single state of Australia, and were not representative of the wider
Australian population.70 The results cannot be confidently generalised to the wider
Australian population. Despite this, the results provide an indication of some of the ways in
which the costs associated with prescription co-payments can influence the quality use of
medicines.
In 2005, the PBS general co-payment increased by $4.90 per prescription (from $23.70 to
$28.60) and the PBS concession co-payment and RPBS co-payment increased from $3.80
to $4.60 per prescription. Such large increases in patient co-payments had not occurred
since November 1990. The impact of the large co-payment increases in 2005 on medicine
affordability for patients remains unclear. Given the results of previous Australian
research, it is possible that these large co-payment changes resulted in decreased medicine
use by patients and this is an area requiring further investigation.
3.1.1 International studies of the effects of increasing patient co-payments
Numerous international studies have considered the effects of increasing patient co-
payments on the use of medicines, and several systematic reviews have summarised the
results.71-74 Similar to the Australian study which looked at changes in the level of
dispensing for essential and discretionary medicines following co-payment changes,68
international studies have considered the impact of cost-sharing increases on the use of
essential and discretionary medicines. One systematic review, which considered the effects
of increasing patient co-payments on the use of prescription medicines and health
outcomes for patients in the USA and Canada,71 found that use of medicines defined as
being essential for the maintenance of health decreased following increases in prescription
33
cost sharing.71 Studies which included both acutely ill and chronically ill patients tended to
find large decreases in essential medicine use associated with increased prescription cost
sharing.71 In contrast, studies in the review which only included chronically ill patients
tended to show smaller decreases in the use of essential medicines with increased cost
sharing,71 suggesting that chronically ill patients may not be as sensitive to price increases
as acutely ill patients. Studies included in the systematic review which considered
individual therapeutic groups of medicine also tended to find smaller differences between
the use of essential and less essential medicines after co-payment changes than studies
which considered aggregate data for all therapeutic groups of medicine defined as essential
or less essential.71 Despite variability in the magnitude of findings, international studies
consistently showed that medicine dispensings decreased following co-payment increases.
Another systematic review found that prescription co-payment increases of 10% could be
expected to lead to a decrease in prescription medicine dispensing of between 2% and
6%.72
A systematic review published in 2007, which aimed to identify the characteristics of
American patients at risk of non-adherence to medicines following co-payment increases,
found that patients without insurance coverage for prescription medicines were at higher
risk of non-compliance due to cost than insured patients.73 Patients who spent more money
on prescription medicines were also more likely to report cost-related non-adherence, even
if they had insurance coverage for prescription medicines.73 This finding was reinforced by
another systematic review, which focussed on the impact of increasing co-payments for the
poor, people with chronic diseases and people with chronic poor health.74 Although health
insurance coverage can help to increase affordability of medicines, when patients use
multiple medicines and therefore have to pay multiple co-payments this can still be a
financial burden. Patients with an annual income of less than US$20,000 and sicker
patients were more likely than richer patients, with an annual income of US$50,000 or
more, to go without their medicines due to the purchase cost.73 Another systematic review
found that patients who were required to pay higher co-payments were more likely to be
non-adherent following co-payment increases.71 A number of studies included in this
review suggested that medication discontinuation is associated with increasing co-
payments, although the extent of discontinuation varied depending on the type of
medicine.71 No systematic reviews found an association between gender, level of
educational attainment, number of medicines used or race and the tendency to go without
34
medicines due to the co-payment cost. However, older patients were less likely to be non-
adherent to their medicines due to the purchase cost than younger patients.73
International studies have also considered the impact of increasing prescription co-
payments on health resource utilisation. These systematic reviews found that, in most
cases, overall hospitalisation rates, emergency department visits, outpatient visits, and GP
consultations were unchanged following co-payment increases.71,72,74 However, studies that
reported the effects of increased cost sharing in patients with specific conditions tended to
find increases in health resource use. In one systematic review, studies that specifically
involved patients with heart failure, high cholesterol, diabetes and schizophrenia, found
that increased co-payments were associated with increased health resource utilisation by
these patients.72 This suggests that the decrease in essential medicine dispensing associated
with co-payment increases has the potential to result in worsened health outcomes for
certain groups of chronically ill patients. One study in a second systematic review found
that there was a decrease in use of essential medicines following co-payment increases,
with an associated increase in health service use by vulnerable patients; however, another
study in the same review which assessed the same co-payment increase but looked at a
single therapeutic group of medicines found no increase in health resource utilisation.74
Other studies in this review, in different populations, found that reductions in the use of
essential medicines by patients following co-payment increases were associated with
increases in nursing home admissions and mental health related hospital admissions.74
There have been a multitude of studies assessing the effects of increasing prescription co-
payments on the use of medicines and health resource utilisation (as a proxy for overall
health status of patients). Although the findings have varied between studies, a consistent
finding in systematic reviews was that use of medicines decreased following co-payment
increases.71-74 The reviews also found some studies that suggested patient health outcomes
may be adversely affected by increases in cost-sharing, with health resource utilisation
increasing as a result.71,72,74 These findings from international studies suggest that increases
in prescription cost sharing can have negative implications for the quality use of medicines.
3.1.2 Patient co-payments - some conclusions
Australian research has shown that consumer demand for government subsidised
medicines decreases in response to co-payment increases. Following co-payment increases
in the 1990s, medicine dispensing decreased.68 Use of discretionary medicines decreased to
a greater extent than essential medicines, suggesting that the changes may have partly
35
achieved their intended effect by prompting patients to reassess and rationalise their
medicine use. Despite this, use of essential medicines also decreased68 and evidence from
other Australian research suggests that quality use of medicines (QUM) may be
compromised by increasing co-payments.70 Patient surveys have highlighted behaviours
non-conducive to quality use of medicines, such as sharing medicines amongst family
members, using medicines prescribed for a prior illness, decreasing the prescribed dose due
to the cost of prescription medicines, or simply not having a prescription dispensed due to
cost.69,70 None of the Australian studies assessed the impact of medicine cessation on
patient health outcomes and health resource utilisation. However, results from international
studies suggest that decreased medicine use associated with increased co-payments can
lead to increased health resource utilisation,71,72,74 an unintended and adverse consequence
of patient co-payments.
Brand premiums increase the cost of medicines to patients. It is possible that the increased
cost of medicines associated with brand premiums could also lead to decreased medicine
use by patients, with the potential for worsened health outcomes, in a similar manner to
increasing co-payments. In the following section of this chapter, the literature surrounding
the PBS minimum pricing policy and brand substitution is reviewed.
3.2 Review of the literature on the PBS minimum pricing policy
and brand substitution policy
The minimum pricing policy applies when there is more than one brand or generic product
available for a medicine. PBS and RPBS medicines are subsidised up to the price of the
lowest priced product(s) of that medicine,1 commonly referred to as the benchmark priced
product(s). Patients who use benchmark priced products pay only the patient co-payment.
Those who use more expensive brands are required to pay the price difference (“brand
premium”) between the benchmark priced and more expensive product, in addition to the
co-payment.1
The minimum pricing policy affects the prices of PBS and RPBS medicines so that the
cheapest medicines are available to patients for the co-payment price and patients who use
more expensive medicines are required to pay a brand premium in addition to the co-
payment. In theory, this price signal should increase demand for and use of co-payment
priced medicines, and demand for and use of premium priced products should decrease.
However, as the review of the literature surrounding patient co-payments showed, patients
may not respond to price signals in the manner expected. It is possible that patients may
36
respond to the increased price associated with brand premiums in a similar manner to co-
payment increases – for example, by not having medicines dispensed, by sharing
medicines amongst family members or by taking less than the prescribed dose. Unintended
and adverse outcomes may occur as a result. The following section of this thesis reviews
research on the effect of the minimum pricing and brand substitution policies on the use of
PBS and RPBS medicines.
McManus and colleagues described changes in dispensing of medicines, both at the
population level and also by individuals, following introduction of the minimum pricing
and brand substitution policies to the PBS.75 Using PBS dispensing claims data, they
compared the proportion of prescriptions dispensed with a brand premium prior to
introduction of brand substitution and after introduction of brand substitution.75 They
found that in the month prior to introduction of the brand substitution policy only 17% of
prescriptions were dispensed at the benchmark price, compared to 45% five years later.75
Using ranitidine and fluoxetine as examples they also looked at the change in dispensing of
benchmark and premium priced products in the months following introduction of generics
and a brand premium. Three months following PBS listing of the first fluoxetine generic, a
$5.06 brand premium applied to the original product.75 Similarly, three months following
PBS listing of the first ranitidine generic, a brand premium was introduced.75 However, the
ranitidine brand premium was only 71c.75 For both medicines, in the three months post
listing of the generic when there was no brand premium the majority of prescriptions were
dispensed for the brand name product.75 This suggests that without the brand premium
price signal there was little incentive for patients to change products. Following addition of
the brand premium, generic fluoxetine dispensing increased and by October 1996 it had
exceeded that of the premium priced product.75 By December 1996, an estimated 55% of
fluoxetine dispensings were for the generic product. Generic ranitidine dispensings also
increased after addition of the brand premium; however, not to the same extent as
fluoxetine. Seven months after addition of the brand premium to ranitidine, it was
estimated that a quarter of ranitidine dispensings were for generic products.75
Patient level analysis showed that of 6,133 patients receiving brand name fluoxetine in
April 1996 only 27% continued to receive the premium priced product in July 1996.75
Thirty nine percent of patients had switched to the benchmark priced product and 3.4% had
switched to other antidepressants.75 The rate of switching to other antidepressants did not
increase after addition of the fluoxetine brand premium.75 Of the 48,000 patients receiving
brand name ranitidine in April 1997, 44% continued to receive the premium priced product
37
three months later, while 8% had switched to generics and 4.3% had switched to other
medicines.75 The rate of switching to other gastric acid suppressing medicines did not
increase after addition of the ranitidine brand premium.75 Presumably the remaining 31%
of fluoxetine patients and 44% of ranitidine patients ceased therapy altogether; however
this was not specified by the authors. It is therefore unknown whether the rate of therapy
cessation changed after addition of brand premiums. This is an important consideration
because cessation may be a potential unintended effect of the minimum pricing policy,
particularly if patients are unaware that they can substitute to benchmark priced products to
avoid the extra cost of the brand premium. The magnitude of the brand premium appeared
to influence switching in this study, with a higher proportion of fluoxetine than ranitidine
patients switching to benchmark priced products.75
McManus and colleagues showed that dispensing of benchmark priced products increased
and that brand substitution occurred following introduction of brand premiums and brand
substitution. Their study used data from soon after introduction of the brand substitution
policy and only considered two medicines for which brand substitution had recently
become possible. The extent of brand substitution for other medicines and for medicines
for which substitution has been possible for longer periods of time remains unknown.
Unintended and adverse outcomes following brand substitution have been described in two
Australian case reports, where patients “doubled up” on brand and generic products
because they did not realise that the different products were actually the same
medicine.76,77 In Australia, generic products are marketed with a unique trade name rather
than just the generic drug name, and product appearance (i.e. tablet shape, colour and
packaging) frequently differs. When multiple brand and generic products are available for
a medicine, patients may be faced with multiple different trade names and products of
different appearance, particularly if brand substitution occurs multiple times.
In the first case report, a general practitioner (GP) described an 18 year old who
inadvertently took a double dose of doxycycline. Two different brands had been dispensed
from the same pharmacy and the patient did not realise the different products were the
same medicine.76 The GP became aware of the double dosing when the patient returned to
the surgery with a skin rash, diagnosed as doxycycline induced photosensitivity.76 In the
second case report, a GP described an elderly patient who double dosed on two different
enalapril products, thinking that they were different medicines because the prescriptions
had been written by different doctors.77 The doctor became aware of the double dosing
when the patient became hypotensive and collapsed. In these cases brand substitution may
38
have contributed to patient confusion and double dosing; however, a similar event was also
reported prior to introduction of the brand substitution policy.78 A 1988 case report
published in the Medical Journal of Australia described a patient who double dosed on two
different propranolol products.78 Because the brand name, tablet appearance and packaging
was different the patient assumed that they were different medicines and that both needed
to be taken.78 This suggests that patient confusion from multiple brand names for the same
medicine can occur even without brand substitution by pharmacists.
Double dosing of different brand or generic products of the same medicine has also been
identified by pharmacists during medicines reviews,13 and is a potential unintended
consequence of brand substitution. Three case reports were presented of patients who had
been recently discharged from hospital and who were followed up post-discharge with a
medicines review.13 Two of the patients were double dosing on different brands of the
same medicine. One of the patients was found to be using two different brands of
frusemide even though the medicine had been ceased during the hospital admission, while
the other patient was using two different diltiazem brands.13 The authors of the paper noted
that brand substitution commonly occurs when patients are discharged from hospital,
which has the potential to result in drug related problems.13 Most Australian public
hospitals have a drug formulary which specifies the medicines that are routinely stocked
and supplied to patients in the hospital. Only one brand or generic product for each
medicine is kept in stock. On discharge from hospital, patients are dispensed up to a weeks
supply of medication.79 If the hospital does not stock the brand of medicine that the patient
used prior to admission, then brand substitution occurs when the discharge prescription is
dispensed.79 No studies in Australia have considered the extent of brand substitution that
occurs when patients are discharged from hospital or the problems that may occur as a
result. However, these case reports highlight the potential for quality use of medicines to
be compromised.
In an Australian study of 204 elderly patients referred for medicines review, it was
reported that 114 (56%) of the patients were confused by brand and generic names of their
medicine.12 Pharmacists identified 22 patients (11%) who had double dosed on different
products of the same medicine.12 The home medicine reviews were conducted by different
pharmacists, so it is possible that different methods to identify or classify confusion with
product names were used by different people. In addition, the study participants were
selected because they were at high risk of medication related problems. It is likely that the
39
actual frequency of confusion from brand substitution across the wider community is lower
than in this study population.
Research with Australian consumers has highlighted the confusion that can arise when
patients are faced with multiple trade names for the same medicine,9,80,81 which can occur
when patients have brand and generic products substituted multiple times. In 1993, the
Australian Pensioners and Superannuants Federation conducted a series of focus group
discussions with older Australians, with the aim of determining their understanding of the
recently introduced minimum pricing policy and their attitudes towards the use of generic
medicines.80 One hundred aged pensioners from English, Polish and Greek speaking
backgrounds and non-pensioner retirees participated. The topic of confusion with different
brand names for the same medicine was discussed. Many patients had difficulty
understanding the difference between different brands of the same medicine and different
medicines altogether.80 This confusion was considered worse for patients who were taking
medicines with many different brands available.80 Consumers also reported confusion in
differentiating between brand names of different medicines in the same therapeutic class,
particularly when there were many medicines within that class used for similar
indications.80 Only a small number of participants in the focus groups (8 out of 104) used
generic versions of their medicine.80 Given that confusion was a common topic for
discussion, it is possible that confusion not only occurs with the substitution or use of
generic products but also with the use of multiple different brand name medicines.
This study was conducted before brand substitution by pharmacists was allowed and soon
after introduction of the minimum pricing policy. It is likely that this had an influence on
the small proportion of patients who actually used generic medicines. Many participants
reported that they had not discussed the cost of medicines or the cost implications of the
minimum pricing policy with their GP.80 Although GPs discussed cost issues relating to
non-PBS medicines with their patients, consumers stated that they did not discuss brand
premiums and reported a lack of understanding on this topic.80 Consumers from non-
English speaking backgrounds had greatest difficulty understanding the cost implications
of the minimum pricing policy.80 The few patients who had discussions with their GP
about the minimum pricing policy were unaware that they did not necessarily have to pay
more for their medicines and that they could request their doctor to prescribe a co-payment
priced product.80
Between 1998 and 2000, the consumer sub-committee of the Pharmaceutical Health and
Rational use of Medicines (PHARM) committee held discussions with consumers and
40
consumer groups representing patients using multiple medicines to determine the common
problems these patients face when managing their medicines.9 Over 110 consumer groups
from city and rural locations and from non-English speaking backgrounds participated in
the research. A total of six focus groups were held.9
The focus groups highlighted the fact that many consumers rely on product appearance
(e.g. tablet colour and shape) to identify their medicines.9 When brand substitution occurs,
the product appearance changes and it was reported that patients had difficulty identifying
their medicine as a result. In addition, it was reported that consumers did not understand
the difference between generic and trade names of medicine.9 When brand substitution
occurred and the trade name of the medicine changed, many consumers thought that the
medicine itself must also be different. It was reported that this had the potential to result in
consumers using two different brands of the same medicine concurrently, or to make the
decision to cease a medicine because it had an unfamiliar trade name and appearance.9
These problems were most commonly reported by elderly consumers, people with poor
eyesight and people with low literacy; and the problem was thought to be more common
since multiple generic products were available for many PBS medicines.9 Lack of
communication from pharmacists regarding brand changes was also reported to contribute
to the uncertainty and confusion experienced by consumers.9 Although this study
highlighted some negative aspects to brand substitution, the findings should be interpreted
bearing in mind the study aims. The focus groups were held to identify problems faced by
consumers using multiple medicines so it is less likely that the positive aspects of brand
substitution, such as cost savings, were discussed.
In 2003, sixteen Australian consumers were interviewed to ascertain their perceptions of
and attitudes towards generic medicines.81 Patients were recruited to the study via emails
and newsletters from a consumer group (n = 10) and through contacts of the researchers (n
= 6).81 Recruitment continued until saturation of themes was reached. Equal numbers of
males and females with a wide range of ages (21 to 80 years old) were interviewed. Eleven
participants suffered a chronic illness and participants used an average of four regular
prescription medicines.81 Unlike the study conducted by the Australian Pensioners and
Superannuants Federation in 1993,80 awareness of generics amongst the consumers in this
study was high.81 An emerging theme from the interviews was the awareness of consumers
that generic medicines were cheaper alternatives of their brand name counterparts.81
Amongst patients who reported using generic medicines, one of the main reasons for doing
so was the lower cost.81 This was particularly common for patients using multiple
41
medicines.81 These findings suggest that although consumer awareness of the minimum
pricing policy was low in the years immediately after its introduction, consumers may now
be better informed on the topic.
Consumers reported using generics on the recommendation of their pharmacist or doctor,
and trust in these healthcare professionals and re-assurance of the safety and therapeutic
equivalence between generic and brand name products influenced acceptance of generics
by these patients.81 In addition, a theme emerging from the interviews was that positive
experiences of friends and relatives who had used generics in the past resulted in
consumers having confidence in their use. Conversely, other consumers reported that they
were less likely to use generics if they had experienced side effects from them in the past.
Others reported that they simply preferred to use the brand of medicine that the doctor
prescribed.81
Some participants in this study voiced concerns that they may become confused by
switching brands. These people described strategies to avoid brand substitution which
included always attending the same pharmacy where the pharmacist knew them and the
product they usually used.81 Some of the interviewees described family members or friends
who had become confused following brand substitution and, in particular, friends and
relatives using multiple medicines or with dementia.81 However, none of the consumers
reported that they had personally experienced confusion as a result of brand substitution.
The authors stated that they tried to recruit patients to the study who had experienced
confusion from brand substitution, but were unable to do so despite repeated attempts. This
may be because the people who are at greatest risk of confusion from brand substitution
are poor candidates for interview research. As the authors discussed, people with cognitive
impairment such as dementia are vulnerable to confusion from brand substitution. These
people are likely to be poor candidates for interview research, particularly if their level of
cognitive impairment is severe. However, an equally plausible reason may be that
confusion from brand substitution is not a common occurrence. As highlighted in this
study, patients who were concerned about becoming confused adopted strategies to avoid
brand substitution and it is possible that other patients in the community use similar
strategies.
The issue of confusion associated with brand substitution also arose in interviews
conducted with pharmacists, where pharmacists highlighted their reluctance to substitute
products for patients they perceived to be at risk of confusion.82 A convenience sample of
eleven community pharmacists from Melbourne, Australia were interviewed in 2005 to
42
determine their perceptions towards generic medicines and brand substitution.82 Study
participants were recruited through personal contacts of the researchers, and by a national
pharmacist professional organisation.82 Recruitment continued until saturation of themes
was reached, with no new themes emerging after the ninth interview.82 A theme expressed
by pharmacists during the interviews was the concern that patients could become confused
and potentially double dose on different generic products of the same medicine, thinking
that they were different medicines because they had different trade names.82 All of the
pharmacists interviewed felt that adequate counselling by pharmacists could help to
minimise patient confusion when brand substitution occurs.82 Despite this, an emerging
theme from the interviews was the reluctance of pharmacists to substitute products for
patients they felt might become confused as a result. The elderly and people from non-
English speaking backgrounds were considered to be most vulnerable to confusion from
brand substitution.82
The pharmacists were generally in favour of recommending generic medicines to their
patients. However, they were less confident in offering substitution when dispensing
medicines for the treatment of chronic conditions.82 In addition, the pharmacists felt that
some patients refused generic substitutes because they preferred to use the brand of
medicine prescribed by their doctor, even if a cheaper product was available.82 The
magnitude of the brand premium – between $1 and $4 for most premium priced PBS
medicines in 20063 – was identified as a barrier to patients accepting generic substitutes.
Many of the pharmacists described customers who were willing and able to pay this
relatively small premium.82
Although only eleven pharmacists were interviewed, this is the only published Australian
study regarding pharmacist opinions towards generics and brand substitution. The
pharmacists recruited through personal contacts of the researchers may not have had views
towards generic medicines and substitution that are representative of all pharmacists. It is
possible that they were selected by the researchers because of their opinions and attitudes
towards generics, although this potential issue wasn’t discussed. The views of the eleven
pharmacists in this study are unlikely to be representative of pharmacists Australia wide.
Despite this, this study provides an indication of the concerns pharmacists have about
brand substitution for certain patients and in certain situations.
Anecdotal reports in Australian medical publications suggest that prescribers are also
concerned about the potential for patient confusion with brand substitution. Concerns have
been expressed by prescribers that patients may have brand and generic products
43
substituted multiple times and that this can worsen the potential for confusion.4-8,83-87 A
media release in 2003 from the Australian Divisions of General Practice (now known as
the Australian General Practice Network) expressed concerns about the potential for
patients to receive a different brand of medicine each time their prescription was
dispensed.4 The current rules of the brand substitution policy do not limit the number of
brand substitutions per patient, medicine or prescription form. On the PBS and RPBS
prescription forms are valid for one year from the date of prescription.2 The standard
dispensed quantity of medicines on the PBS and RPBS usually equates to one months
supply. When continuing therapy is required the prescriber may order the original one
months supply and up to five one month repeats; equating to approximately six months
supply of medicine at standard doses related to one prescription form. The Australian
Divisions of General Practice expressed concern that
− “for patients who have a script with five repeats this can mean receiving six
different versions of the same drug over the life of the script.”4
They suggested that a limit of one brand substitution per prescription form should be
enforced to reduce the likelihood of patient confusion that may occur if there are multiple
brand substitutions per prescription.4
A study conducted in 1995 by Australian Doctor magazine surveyed 71 general
practitioners (GPs) by telephone. The survey found that approximately equal numbers of
GPs were for (31%), against (39%) or had mixed feelings (30%) towards brand
substitution.88 Doctors who were against brand substitution commonly reported concerns
about the potential for patient confusion with brand substitution and the potential negative
impact that this could have on health outcomes.88 This was also reported by doctors who
expressed mixed feelings towards the use of generics; however, many of these doctors also
acknowledged the financial benefits to their patients associated with brand substitution.88
This was a recurring theme amongst doctors who supported brand substitution, who most
commonly reported doing so because of the potential cost savings for their patients.88 The
survey only covered a small proportion of Australian doctors and was conducted less than
six months after generic substitution was first allowed. The survey response rate was not
reported. The small number of survey respondents and the absence of a response rate make
the quality of the study unclear. Given the small sample size, the results may not be
generalisable.
Concerns regarding patient confusion from brand substitution were also highlighted in
prescriber interviews. In 2005, interviews were conducted with a convenience sample of
44
ten GPs in Melbourne, Australia using a semi-structured questionnaire.89 The aim of the
research was to determine the factors which influence prescribing of generic medicines.
Advertisements were placed in a Melbourne GP newsletter inviting interested doctors to
participate; recruitment continued until saturation of themes was reached.89 All of the
participating GPs practised in suburban or inner city locations. Several themes emerged
from the interviews. With regards to generic prescribing, the GPs were either pro-generics
or anti-generics.89 GPs who were anti-generics tended to cite the potential for patient
confusion with brand substitution as reasons for their oppostion.89 In addition, some
respondents felt that brand substitution equated to the government telling them what they
should prescribe, while others opposed the use of generics out of support for drug
companies who invest in the research and development of new medicines.89 GPs who were
pro-generics cited cost savings to both patients and the health system as reasons for
supporting their use.89 Most of these doctors felt that their patients would accept a generic
substitute for this reason. In addition, GPs who were pro-generics felt that it was more
confusing to prescribe by brand name because of the multitude of brand names for the
same medicine.89 A theme emerging from the interviews was that some GPs perceive
medicine labelling requirements in Australia to be inadequate, particularly when brand
substitution occurs.89 They suggested that it should be a requirement for the generic
medicine name to be more prominent than the brand name on the pharmacy dispensing
label.89
Although the sample size for this study was small, the findings were similar to the 1995
study conducted by Australian Doctor magazine. In both studies, some doctors expressed
concern about the use of generics and brand substitution because there is potential for
patient confusion from brand substitution, while other doctors expressed support for brand
substitution because it could save their patients money. The brand substitution policy has
provisions for prescribers to prevent substitution, by designating the prescription “brand
substitution not permitted”. Neither of these studies determined the extent to which
prescribers who have reservations towards brand substitution prevent it from occurring by
so designating the prescriptions they write.
The most recent Australian research regarding GPs prescribing habits and opinions
towards generic medicines was conducted by the Australian Medical Association (AMA)
in April 2006. A survey was conducted in response to anecdotal reports that pharmacists
substituted products even when the prescriber had designated the prescription “brand
substitution not permitted”. The aim of the study was to determine the extent of brand
45
substitution and the opinions of prescribers towards brand substitution.90 Survey forms
were faxed to 1,508 GPs and 386 (26%) completed the survey. When asked how often they
marked prescriptions “brand substitution not permitted”, 80% of respondents reported
doing so for less than 25% of prescriptions. Only 12% of GPs reported that they prohibited
substitution for more than 75% of their prescriptions.90 The most common reason for
precluding substitution was the risks to patient safety associated with brand substitution
(70% of respondents),90 although the nature of these risks was not described. Sixty five
percent of doctors felt that patient compliance may be negatively impacted by brand
substitution, while a quarter reported that they precluded brand substitution due to the
small cost difference between brand and generic products.90 Eight GPs described situations
where patients had double dosed on two brands of the same medicine following brand
substitution and one GP reported that their patient had been hospitalised as a result.90 This
is the largest Australian study of prescribers regarding their opinions towards brand
substitution. Although many GPs expressed concerns about brand substitution, the majority
reported that they did not prohibit brand substitution for the majority of prescriptions they
wrote.
This finding was supported by an email survey of 1,700 pharmacists conducted by the
Pharmaceutical Society of Australia (PSA) in June 2006. Of 312 respondents (18%
response rate), 72% reported that less than 20% of prescriptions they dispensed had brand
substitution prohibited by the doctor.91 Over 90% of pharmacists reported “regularly”
offering brand substitution to their customers.91,92 However, the definition of “regularly”
was not described and the actual frequency of brand substitution was not discussed. More
detailed estimates of the extent of brand substitution by pharmacists for a wider range of
the Australian community have not been reported. Despite this, the findings of this survey
in conjunction with the survey conducted by the Australian Medical Association90 suggest
that brand substitution is possible for the majority of government subsidised prescriptions
dispensed from community pharmacies in Australia. Although many prescribers have
expressed concerns about the potential for multiple brand substitutions per patient and the
confusion that supply of multiple different products may cause, they don’t tend to prevent
substitution for most of the prescriptions they write. There is a large amount of anecdotal
evidence regarding prescriber concerns that patients have brand and generic products
substituted multiple times and the potential for confusion that this presents;4-6,8,83-87,89
however no studies have been conducted to determine the extent of multiple brand
substitutions in Australia.
46
3.2.1 International studies of brand substitution
Brand substitution policies exist in many countries including Denmark, Germany, the
Netherlands, Poland, France, Italy, Portugal, Norway, Spain, Sweden, the United States
and Canada.93-95 Despite the large number of countries where brand substitution by
pharmacists is allowed, there are only a handful of publications regarding the extent of
brand substitution of products.
A study was conducted in the USA following introduction of the first fluoxetine generic to
determine the extent of uptake of the new generic product.95 Fluoxetine is a selective
serotonin re-uptake inhibitor (SSRI) antidepressant. The original brand name product had
the largest market share of any antidepressant in the USA prior to its patent expiry and was
the fifth most prescribed medicine overall.95 Using prescription claims data from a large
pharmacy insurance organisation, fluoxetine dispensings were studied in the five month
period after the first generic became available.95 The prescription claims data covered
patients from many states of the USA, some of which had laws requiring generic
substitution if a generic product was available.
Two weeks after its introduction, 70% of fluoxetine dispensings were for the new generic
product.95 Over the five months of follow-up, 75% of patients who had been using the
original brand name product switched to the fluoxetine generic and 66% of patients who
were initiated on antidepressant therapy with fluoxetine received the generic.95 There was
no change in the rate of switching from other antidepressants to fluoxetine after
introduction of the generic compared to the three months prior.95 A number of factors were
identified that were associated with the tendency to initiate therapy with generic fluoxetine
or to switch from the original brand to the generic. New fluoxetine users were more likely
to receive the generic if their out of pocket medicine payment was more than $20 (odds
ratio 1.26; 95% CI 1.20 - 1.31) and existing users with out of pocket costs of more than
$20 were also more likely to switch to the generic than other patients (hazard ratio 1.40,
95% CI 1.34 - 1.47).95 This compares to the Australian study by McManus et. al., where a
higher proportion of patients switched to a co-payment priced product when there was a $5
brand premium compared to patients using a product with a 70c brand premium.75 New
fluoxetine users who lived in states where brand substitution by pharmacists is compulsory
were more likely to receive the generic than the brand name product (odds ratio 1.28, 95%
CI 1.25 - 1.31). Existing fluoxetine users in states with mandatory generic substitution
policies were also more likely to switch from the brand name to the generic product than
patients living in states where generic substitution was not compulsory (hazard ratio 1.28,
47
95% CI 1.25 - 1.31).95 This study did not report repeat brand substitutions for patients, so
the extent of multiple brand substitutions per patient is unknown. In addition, fluoxetine
dispensings were only followed for five months after introduction of the generic. It is
unclear whether the high rate of generic dispensing was maintained over a longer time
period.
Brand substitution of medicines was introduced in Sweden in October 2002.94 Following
introduction of the policy, pharmacists were required to dispense the cheapest
bioequivalent product available unless the prescriber had prohibited brand substitution of
that prescription, or if the patient refused the cheapest product.94 A study was conducted
with the aim of determining barriers to acceptance of generic substitution by prescribers,
pharmacists and patients.94 Analysis of 501,400 prescriptions for diclofenac, enalapril,
aciclovir, doxycycline, citalopram and simvastatin dispensed in the year following policy
introduction found that brand substitution was allowed by the prescriber for over 90% of
prescriptions.94 Although the proportion of patients who refused generic substitutes varied
amongst the different study medicines, it was generally low, and on average fewer than 5%
of aciclovir, doxycycline, citalopram and simvastatin patients refused generic substitutes.94
A quarter of enalapril patients and 15% of diclofenac patients refused generic substitutes.94
Although prescribers allowed generic substitution for the majority of prescriptions and
patients accepted substitutes in the majority of cases, the actual extent of brand substitution
was lower, with on average between 15% and 70% of prescriptions for each of the study
medicines dispensed for the cheapest generic product.94 This was particularly the case for
enalapril, citalopram and simvastatin, where on average only 15%, 25% and 35% of
prescriptions, respectively, were dispensed for the cheapest product available.94 It is likely
that a large barrier to substitution occurring was availability of generic products.
Substitution to the cheapest generic was not possible for on average over half of
doxycycline dispensings and approximately 40% of enalapril, citalopram and simvastatin
dispensings because the cheapest product was out of stock.94
Manufacturers of products were able to change their prices during the study period,
meaning that the cheapest product available for some medicines frequently changed.94
Medicines with few price changes tended to have the highest proportion of prescriptions
dispensed for the cheapest product over the year of follow-up.94 For example, the price of
enalapril changed numerous times during the year of follow-up and the overall proportion
of enalapril prescriptions dispensed as the cheapest product was low (15%). In the months
when the original brand name enalapril product was the cheapest available, a high
48
proportion of prescriptions were dispensed for this product.94 In contrast, when the price
changed and newer enalapril products were the cheapest available, fewer prescriptions
were dispensed for the cheapest available product.94 These findings suggest that patients
exhibit some degree of brand loyalty, and perhaps tend to continue using the same product
even if the price changes and it is no longer the cheapest available. However, patient level
data were not used for this study, so the extent to which individuals switched products as
the cheapest available product changed and the extent to which individuals remained brand
loyal was not determined.
The authors of this Swedish study noted that pharmacists were required to dispense the
cheapest product available for a medicine unless the patient or prescriber opposed this, and
that the cheapest product for some medicines frequently changed as manufacturers altered
their prices.94 It is possible that some patients had multiple different products supplied
during follow-up. Parallel import products (that is, products manufactured for use in a
country other than Sweden) were allowed to be substituted if they were the cheapest
product available.94 Parallel imports frequently have a different appearance to other
products of the same medicine and the labelling may be in a foreign language.96,97 The
potential for patient confusion from multiple brand substitutions of products of different
appearance was not discussed or assessed in this study. It is possible that this was not yet
identified as a potential issue because brand substitution had only been possible in Sweden
for a year.94
In the Netherlands reference pricing is used to set a threshold for reimbursement of
medicines that are therapeutically interchangeable.33 When new generics enter the market,
many manufacturers set their prices near to the reimbursement threshold, which has the
potential to limit cost savings to the government from introduction of generics.33 It may
also mean that patients have little financial incentive to switch from brand name to generic
products, because there may be only a small price difference.33 This is similar to the
situation in Australia, where most generic products are only $1 to $4 cheaper than the
brand name alternative3 and some patients are willing to pay this relatively small extra
charge.82 Brand substitution by pharmacists is permitted in the Netherlands. Using
dispensing data from an administrative claims database, a study was conducted to
determine changes in medicine dispensing after introduction of generics for ranitidine,
enalapril and fluoxetine. Dispensings for all three medicines were studied over the six year
period from the start of 1996 until the end of 2001. The ranitidine patent expired in 1996
and the first generic products became available in mid-1997. The patents for both enalapril
49
and fluoxetine expired in 1999 and by the end of that year generic products were available
for both medicines.33
A year after introduction of the first ranitidine and fluoxetine generics, around three
quarters of prescriptions dispensed for each medicine were for the generic products.33 A
year after introduction of the first enalapril generic, nearly 90% of prescriptions were
dispensed for generic products.33 This high level of generic dispensing was maintained for
all three medicines in the following year. Patients initiating therapy with enalapril or
ranitidine almost always received a generic, with 99% and 97% of patients respectively
receiving generic products. The figure was lower for fluoxetine, with 79% of patients
initiated on a generic.33 Brand substitution at the patient level was not considered in this
study. Given the long period of follow-up, it is possible that some patients received
multiple different generic products over the study period, or that some patients substituted
to a generic but then reverted back to the original brand name product. However, the extent
to which this occurred was not reported.
In another study conducted in the Netherlands, medicine dispensing following patent
expiry of omeprazole was studied.98 Data from an administrative claims database was used,
looking at the four year period from 2000 to 2003. The omeprazole patent expired in early
2002. In the first three months following patent expiry, 60% of dispensings were for the
brand name product.98 A year after introduction of the first generic, this had reversed and
60% of dispensings were for generic products.98
Two cohort studies were conducted by the same authors to determine whether patients
were more or less likely to switch from omeprazole to other proton pump inhibitors (PPIs)
after patent expiry. Their rationale was that if patients were more likely to switch from
omeprazole to other PPIs after omeprazole patent expiry, then the cost savings to the
government from increased use of omeprazole generics would be diminished.98 In the year
before omeprazole patent expiry 8% of patients switched from omeprazole to other PPIs;
most commonly pantoprazole. In the year after omeprazole patent expiry 14% of patients
switched to other PPIs; a statistically significant increase (p<0.001).98 After patent expiry,
patients were most likely to switch from omeprazole to esomeprazole.98 Esomeprazole was
introduced to the pharmaceutical market in the Netherlands in late 2000.98 The authors
commented that the increase in PPI switching may have occurred simply because
esomeprazole entered the market place and that it may have had nothing to do with
omeprazole patent expiry.98 However, the manufacturers of the original brand name
omeprazole product also marketed esomeprazole.99 The drug company implemented a
50
worldwide marketing campaign prior to introduction of generic omeprazole to encourage
prescribers to switch patients from omeprazole to esomeprazole.100 This marketing
campaign may have influenced the results of this study. It is likely that the potential cost
savings from omeprazole generics were diminished due to the high rate of patients
switching from omeprazole to esomeprazole following omeprazole patent expiry.
The authors of this study had access to patient-level information, and although the extent
of switching between different proton pump inhibitors was studied at the patient level, the
extent of brand substitution per patient was not studied. Although it is possible that
individual patients received multiple different omeprazole generics during follow-up, or
that they substituted to the generic but then switched back to the brand name product, the
extent to which this occurred was not studied by the authors.
A study was conducted in America to identify factors associated with patients initiating
therapy with generic rather than brand name medicines and to identify factors associated
with patients switching from brand name to generic medicines after they had initiated
therapy using brand name products.101 Patients who were continuously enrolled in a single
American health insurance plan from October 2001 until October 2002 were eligible for
inclusion, if they were new users of calcium channel blockers, statins, oral contraceptives,
angiotensin converting enzyme (ACE) inhibitors, histamine-2 (H2) receptor antagonists or
proton pump inhibitors (PPIs). These drug classes were chosen because they are frequently
dispensed and are generally used in the treatment of long term conditions.101 Multiple
brand name and generic alternatives were available for each of the drug classes.
The first dispensing of a study drug to included patients between October 1st 2001 and
October 1st 2002 was identified as being either a brand or generic product. Patients initially
dispensed a brand name product were defined as having switched to a generic if they were
dispensed a generic product in the year following their initial supply.101 Multivariate
analysis was conducted to determine the influence of patient age, gender, income, number
of prescription medicines used; prescriber age, gender and specialty; whether the initial
supply was dispensed at an independent, chain or mail-order pharmacy; and the level of
prescription co-payment required by the patient’s health insurance plan on the likelihood of
initiating therapy with a generic medicine.101 The same variables were included in a
multivariate model to determine factors associated with switching to a generic after having
been initiated on a brand name product.101
51
Five thousand three hundred and ninety nine patients were included in the study. The
majority were female (60%), aged between 36 and 55 years (68%) and had a health
insurance plan with three tiers of co-payments for prescription medicines (93%).101
Overall, the majority of patients initiated therapy with a brand name product (76%).101
Multivariate analysis showed that, compared to patients with a low annual income of less
than $30,000, richer patients were more likely to receive a generic medicine at their initial
dispensing (relative risk 1.28 (95% CI 1.06 – 1.56), p = 0.01 for patients with annual
incomes of $30,000 to $60,000; and relative risk 1.29 (95% CI 1.04 – 1.60), p = 0.02 for
patients with an income of more than $60,000).101 The number of prescription medicines
used, type of dispensing pharmacy (independent, chain or mail-order), the level of
prescription co-payment required by the health insurance plan and physician age were not
associated with the type of product received by patients at their initial dispensing. The type
of prescribing doctor was associated, with obstetricians less likely than general
practitioners to initiate patients on generic products (relative risk 0.81 (95% CI 0.69 –
0.95); p = 0.01).101 Age was inconsistently associated. Men aged less than 25 years were
more likely to receive generics at their initial dispensing than other males (p < 0.001 for all
age group comparisons). In contrast, women aged under 25 were less likely to initially
receive a generic than women aged 25 to 39 (relative risk 1.36 (95% CI 1.15 – 1.61); p <
0.001) and all other age group comparisons were insignificant.101
Of the patients who initiated therapy with a brand name product, 15% switched to a
generic in the year of follow-up.101 For men, age was not associated with the tendency to
switch from brand name to generic medicines.101 In contrast, females aged 25 years or
under were less likely to switch from brand name to generic products than females in all
other age groups (p ≤ 0.002).101 Patient income, number of prescription medicines used and
prescribing physician age and specialty were not associated with switching from brand
name to generic medicines.101 Compared to patients with only one or two tiers of
prescription co-payments in their health insurance plan, patients with three or four co-
payment tiers were more likely to switch from brand name to generic products (p ≤
0.03);101 suggesting that cost is a motivating factor to switch. Patients who received
medicine dispensed at a mail order pharmacy were also more likely to switch from the
brand to a generic product (relative risk 1.65 (95% CI 1.18 – 2.30); p = 0.03).101
This is the only published research located which has examined factors other than cost
associated with the tendency for patients to switch from brand name to generic products.
The issue of multiple product changes and the potential for patient confusion as a result
52
were not considered in the research. The patients included in the study were relatively
young, and the sample was drawn from a single health insurance organisation in America.
Results of the research may not be applicable to different populations or older age groups.
Despite this, the research provides an indication of the situations in which brand
substitution is likely to occur.
The study highlighted the fact that few patients were initiated on therapy using generic
medicines and few patients subsequently switched from brand name to generic
medicines.101 This may have implications for the affordability of medicines, particularly
because patients in the lowest income range were more likely than others to receive brand
name medicines at their initial dispensing and were no more likely than others to
subsequently switch to generic products.101 It is likely that patients in lower income
brackets were least able to afford the extra cost associated with brand name medicines and
if this is the case, there is the potential for quality use of medicines to be compromised if
receipt of brand name medicines means that patients cannot afford to buy their medicines.
This was reflected in a study of patients whose prescription coverage included only generic
medicines. Nearly half of the patients took less than the prescribed dose and a third ceased
medicine use altogether when they were prescribed medicines with no generic
alternatives.102 The extra cost of brand name medicines compared to generic medicines has
the potential to lead to behaviours non-supportive of quality use of medicines. The study
was not designed to determine why poorer patients were less likely than others to receive
cheaper generic medicines and this is an area requiring further research. Given the
relatively young age of patients included in the study and the fact that patients were from a
single health insurance organisation, this finding may not be applicable to other
populations.
Using pharmacy claims data from the Ontario Drug Benefit formulary, a study was
conducted to compare the rates of switching back from generic to brand name products for
patients using anti-epileptic medicines and patients using other medicines for the treatment
of long-term conditions.103 In Ontario, Canada, generic substitution for off-patent
medicines subsidised on the provincial drug benefit plan is compulsory unless the
prescribing doctor submits a “letter of medical necessity” to allow subsidy of the brand
name version.103 The study aimed to identify “switchback” rates from generic to brand
name versions of the anti-epileptic medicines lamotrigine, clobazam and valproate;
compared to “switchback” rates for simvastatin (a statin medicine used for the treatment of
hypercholesterolaemia), and the selective serotonin re-uptake inhibitor (SSRI)
53
antidepressants fluoxetine and citalopram. “Switchback” was defined as when a patient
was switched from the brand name to the generic version of one of these medicines, and
then switched back again to the brand name product.103
Patients were eligible for inclusion in the study if they received consecutive dispensings of
a study drug no more than sixty days apart for at least three months prior to generic
availability and if they were switched from the brand name product to the generic when the
generic became available.103 The study period began one year prior to generic introduction
for the study drugs (in 1995 for valproate and fluoxetine, 1998 for clobazam, 2002 for
lamotrigine and simvastatin and in 2003 for citalopram) and ended in March 2006.103 Only
one brand name product was available for each of the study drugs and there were five
lamotrigine generics, six clobazam generics, fifteen valproate generics and more than ten
generic versions each for simvastatin, fluoxetine and citalopram.103
Results of the study showed that a higher proportion of patients using the antiepileptic
drugs switched back to the brand name product than patients using the other study drugs.
Twenty one percent of valproate and clobazam users and 13% of lamotrigine users
switched back to the brand name product, compared to only 2% of simvastatin and
citalopram users and 3% of fluoxetine users.103 The authors concluded that the higher
switchback rates for antiepileptic medicines compared to the other study drugs were
associated with patient anxiety regarding potential or actual loss of seizure control
following brand substitution, and experiencing side effects with the generic versions.103
They also concluded that these findings support the results of prior research which has
shown that generic antiepileptic medicines may have lower efficacy and more side effects
than the original brand name versions.103
However, the data presented in the paper does not support such strong conclusions. The
average length of follow-up and/or number of medicine supplies to patients using the study
drugs was not reported. It is therefore unclear whether patients using the antiepileptic drugs
had similar opportunity to switchback to the brand name product as patients using the other
study drugs. Previous research has shown that nearly 75% of patients initiated on
lamotrigine continued to receive lamotrigine at two years of follow-up.104 In contrast, in
prior research only 38% of SSRI users continued to receive the medicine eight months
after initiating therapy105 and only 50% of statin users continued to receive statin therapy
one year post initiation.106 These findings from prior research suggest that patients using
antiepileptic medicines continue therapy for longer duration than patients using the other
study drugs. Longer duration of use is likely to be associated with greater opportunity to
54
switchback to brand name medicines, so if users of antiepileptic medicines had longer
duration of follow-up this is a potential explanation for the higher switchback rates for
antiepileptic users than patients using the other study drugs. In addition, inclusion criteria
for the study required patients to switch from the brand name product to the generic;
however, included patients were not required to have another medicine dispensing
following the initial supply of the generic product. It is possible that some included
patients did not continue therapy following the initial switch from the brand name to the
generic product, and therefore did not have the opportunity to switch back to the brand
name product. The study was funded by the manufacturer of brand name lamotrigine who
likely lost a large amount of market share for their product following introduction of
lamotrigine generics, due to the mandatory generic substitution policy in Ontario. This may
also have influenced their negative conclusions towards generic antiepileptic medicines.
Despite these limitations, this is the only published study located which has considered the
extent to which patients switch between brand and generic products more than once.
Switching between different generic versions of the study drugs and the potential for
patient confusion as a result was not considered, despite availability of multiple generic
alternatives for all of the study drugs. International research has noted the difficulty in
continuous supply of the same generic product to patients if pharmacies change generic
supplier, or if patients have their medicine dispensed at multiple different pharmacies.107 It
is therefore likely that some patients included in this study received multiple different
generic products of the same medicine, however, the extent to which this occurred remains
unknown.
Unintended and potential adverse consequences of brand substitution were not discussed in
quantitative international research. Although concerns regarding the potential for patient
confusion from brand substitution have been highlighted in qualitative Australian research
with prescribers, only one international survey of prescribers was located which also
uncovered this concern. In 1990, the research unit of the Department of General Practice at
the University of Otago in New Zealand conducted a survey of GPs to determine their
attitudes towards generic substitution.108 At the time the survey was conducted, the New
Zealand government was proposing the introduction of brand substitution by
pharmacists.108 Surveys were sent to 200 doctors and a response rate of 91% was achieved.
The high response rate was likely due to the fact that the survey population was a group of
GPs who had indicated their willingness to participate in research. Doctors who were
55
newly registered were over-represented in the sample in comparison to the general
prescriber population.108
Half of those surveyed were against the introduction of brand substitution by pharmacists,
while 24% agreed with the proposal and the same number were undecided.108 Two thirds
of doctors surveyed reported that they had patients who had “problems” associated with
the use of generic medicines.108 The most commonly reported problem, described by 90%
of these doctors, was patient confusion arising from the differing appearance of substituted
medication. Other reported problems included side effects with the generic or perceived
lack of efficacy of the generic.108
3.2.2 Brand substitution policies - some conclusions
The available Australian data suggests that the price signal associated with the minimum
pricing policy has led to increased use of co-payment priced medicines, and that following
introduction of brand substitution more patients responded to the price signal and switched
to using co-payment priced products.75 However, data for only two medicines were
studied, the follow-up time was short and the research was conducted soon after
introduction of brand substitution in Australia. The extent of brand substitution for other
medicines, over longer periods of time, is unknown. International studies have also shown
that the use of generics increases after patent expiry.33,95,98 The increase in use of generics
was, in some cases, in response to the price difference between generic and brand name
products.95
When Australia’s brand substitution policy was originally planned and implemented, it was
intended to facilitate access to medicines by enabling patients to switch from medicines
with a brand premium to cheaper, co-payment priced products. Results of Australian
research with consumers,81 pharmacists,82 and prescribers89 suggest that the brand
substitution policy is an important mechanism to increase the affordability of medicines.
Introduction of the brand substitution policy was not intended to facilitate multiple repeat
switches between multiple different products. Prescriber,88-90 pharmacist82 and
consumer9,80,81 surveys conducted in Australia all highlighted the potential for patient
confusion from multiple brand substitutions. Anecdotal reports suggest that prescribers are
concerned that patient confusion may be worse when brand substitution occurs multiple
times and that there may therefore be a need to limit the number of brand substitutions per
patient or prescription.4,5 Despite the widespread belief that patients can be confused by
brand substitution and the concerns that patients have products substituted multiple
56
times,4-8,83-87 the actual number of brand substitutions per patient remains unknown. No
research has been published on the topic.
No international studies were located which have assessed prescriber, pharmacist or
consumer concerns regarding the potential for patient confusion from multiple brand
substitutions or the extent to which multiple brand substitutions occur. It is unclear why
this is the case. In the USA generic alternatives tend to be marketed under the generic
medicine name rather than a unique trade name,109 however, different generic versions of
the same medicine may have different appearance. If patients rely on tablet appearance to
identify their medicines, then supply of multiple different generic products of different
appearance has the potential to confuse patients. In New Zealand there is only one product
available for many government subsidised medicines,110 however, in the past the
subsidised product for some medicines has frequently changed.111 When this occurs,
patients must switch products in order to receive their medicine at the subsidised price,
with the potential for confusion as a result.111 Brand substitution by pharmacists is not
permitted in the United Kingdom (UK), however there is the potential for different generic
products to be dispensed consecutively if a prescription is written by generic medicine
name.93 In this situation, the pharmacist may dispense any generic product, so there is
potential for patients to receive multiple different products of the same medicine. There is
also the potential for patient confusion from dispensing of parallel imported products
which are treated like generics, and which may be of different appearance or may be
labelled in a foreign language.96,97 Parallel imports are sold in many European countries,
and brand substitution is possible in many European countries.93 Despite the paucity of
published research on the topic, it is likely that the potential for patient confusion from
multiple brand substitutions is not an issue isolated to the Australian setting.
Confusion from multiple brand substitutions has the potential to contribute to double
dosing or therapy cessation,9 outcomes that are clearly at odds with the quality use of
medicines. The extent to which there are multiple brand substitutions per patient and the
frequency with which this causes confusion and compromised quality use of medicines is
unknown.
3.3 Review of the literature on the PBS therapeutic group
premium policy
The therapeutic group premium policy applies to medicines within a therapeutic class
which are of such similar safety and efficacy that they can be interchanged without any
57
expected change in therapeutic outcomes.59,63 The lowest priced medicine within each
therapeutic group sets the “benchmark” price for other medicines.59 Manufacturers of other
medicines within the group must reduce their price to the benchmark price or a therapeutic
group premium will apply. This is the price difference between the benchmark priced and
more expensive medicine. The therapeutic group premium policy affects the prices of PBS
and RPBS medicines in a similar way to the minimum pricing policy. Theoretically, if the
therapeutic group premium policy has its intended effects, the dispensing of co-payment
priced products should increase compared to before the introduction of the policy and the
dispensing of premium priced products should decrease by a similar amount.112
Government costs for medicines within the therapeutic groups affected by therapeutic
group premiums should decrease.112
The impact of the introduction of therapeutic group premiums was studied with interrupted
time series analysis using PBS and RPBS administrative claims data.112 Dispensings for
histamine-2 (H2) receptor antagonists, dihydropyridine calcium channel blockers and ACE
inhibitors between January 1994 and January 2000 were studied. Therapeutic group
premiums were introduced for each of these therapeutic groups in February 1998.
For the H2 receptor antagonists, there was a trend of increasing dispensings each month for
the premium priced medicine prior to introduction of the therapeutic group premium.112
After the policy was introduced the trend switched from an increase of over 1,300
prescriptions dispensed each month, to 772 fewer prescriptions dispensed each month for
the premium priced medicine (p < 0.0001).112 The level of the series dropped by 39,314
prescriptions (p < 0.0001).112 Although the level of the series for dispensing of benchmark
priced H2 receptor antagonists increased by 18,897 prescriptions after introduction of the
therapeutic group premium (p = 0.003), the overall trend in dispensing was unchanged.
Prior to introduction of the policy there was an increasing trend of 569 prescriptions per
month for the benchmark priced medicines, and the trend was for 684 extra scripts
dispensed each month post introduction of the policy (p = 0.07).112 Overall dispensing for
H2 receptor antagonists decreased slightly following introduction of therapeutic group
premiums.112 Prior to introduction of the policy, there was an increasing trend of nearly
2,000 prescriptions per month; however, the trend reduced to 639 extra scripts each month
post introduction of the policy (p = 0.01) and the overall level of the series for H2
antagonist dispensing decreased by 16,490 prescriptions (p = 0.06).112 There was also a
significant decrease in government costs for H2 receptor antagonists following
introduction of the policy.112 Prior to introduction of the policy, the trend was for
58
government expenditure on H2 receptor antagonists to increase by around $30,000 each
month; however after introduction of therapeutic group premiums the trend was for
government expenditure to decrease by $28,000 each month (p < 0.0001).112
The implications of the findings for the H2 receptor antagonists are unclear. It is possible
that introduction of therapeutic group premiums resulted in decreased use of H2 receptor
antagonists, which could have potentially negative implications for quality use of
medicines. However, it is also possible that the decrease in H2 receptor antagonist
dispensing was less related to introduction of the therapeutic group premiums than to
increased availability and use of proton pump inhibitors (PPIs) in the Australian market.
Initially, the PBS listing for PPIs required prior approval for supply; however in 2001 the
prior approval requirement was removed.113 Aggregate dispensing data from Medicare
Australia shows that, over the study time period, PBS dispensings for PPIs increased from
301,459 prescriptions in 1994 to nearly 3.5 million prescriptions in 2000.114 The decrease
in H2 receptor antagonist use may have been associated with the increased use of PPIs in
Australia that occurred at a similar time to introduction of the therapeutic group premium.
Following introduction of therapeutic group premiums to ACE inhibitors use of the
premium priced medicine decreased, with a drop in the level of the series of 48,569
prescriptions (p < 0.0001). Prior to introduction of therapeutic group premiums there was
an increasing trend of 279 dispensings each month for the premium priced product;
however, this reverted to a decreasing trend of 1,754 prescriptions each month after
introduction of the extra charge (p < 0.0001).112 Although there was a significant rise in the
level of the series for benchmark priced ACE inhibitors after introduction of the
therapeutic group premium of 21,217 prescriptions (p < 0.001), the overall trend for
dispensing of co-payment priced ACE inhibitors did not change.112 There was a trend of
increased dispensing of co-payment priced ACE inhibitors of 2,090 prescriptions each
month prior to introduction of therapeutic group premiums compared to 1,905 extra
prescriptions each month after (p = 0.58).112 Overall use of ACE inhibitors decreased after
introduction of the therapeutic group premium. There was a decrease in the level of the
series of 59,237 prescriptions after introduction of the therapeutic group premium (p =
0.0003); and the dispensing trend reduced from an extra 7,129 prescriptions dispensed each
month prior to the policy to 2,309 extra prescriptions each month after (p < 0.0001).112
Government spending on ACE inhibitors also decreased after introduction of therapeutic
group premiums. Prior to introduction of the policy, government costs were increasing by
59
around $150,000 each month, compared to an increase in costs of only $11,000 each
month after introduction of therapeutic group premiums (p < 0.0001).112
The situation for calcium channel blockers was less clear. Prior to introduction of the
therapeutic group premium, the trend was for 3,843 extra scripts dispensed each month for
the premium priced product.112 This decreased to 1,005 extra scripts dispensed each month
after introduction of the extra charge (p < 0.0001).112 The level of the series for dispensing
of the premium priced calcium channel blocker dropped by 47,364 prescriptions. The level
of the series for dispensing of benchmark priced calcium channel blockers also decreased
after introduction of therapeutic group premiums, by 15,713 prescriptions (p = 0.002).112
The underlying trend for dispensing of benchmark priced calcium channel blockers was
unchanged (p = 0.45). Overall, the use of all calcium channel blockers decreased after
introduction of therapeutic group premiums. Prior to introduction of therapeutic group
premiums, there was a trend of 4,272 extra prescriptions dispensed each month; this was
reduced to an increase of 1,675 prescriptions each month after introduction of the policy (p
< 0.0001).112 The overall level of the series for dispensing of calcium channel blockers
decreased by 52,576 prescriptions (p < 0.0001).112 There was a significant decrease in
government costs for calcium channel blockers after introduction of the therapeutic group
premium, with the trend changing from an increased cost of $82,000 each month prior to
the policy to an increase of $26,000 each month after (p < 0.0001).112
Although introduction of the therapeutic group premium policy in Australia resulted in
decreased use of premium priced medicines, use of benchmark priced medicines did not
increase to the same extent. This suggests that some patients who were using medicines in
the therapeutic groups affected by the therapeutic group premium policy ceased therapy
with these medicines. It is unclear how many of these patients ceased therapy altogether
and how many patients switched to similar products in other therapeutic groups. Patient
level data were not available for this study, so the number of patients who switched or
ceased therapy as a result of introduction of therapeutic group premiums is unknown.
Similarly, the health consequences for the patients who ceased all therapy are also
unknown. This is an important consideration because, although the therapeutic group
premium policy resulted in decreased medicine costs for the government, there is the
potential for this saving to be offset by the costs associated with increased health resource
utilisation from therapy cessation or switching.
60
3.3.1 International studies of reference pricing policies
Reference pricing policies in Canada and New Zealand are similar to the therapeutic group
premium policy on the PBS, in that they apply to medicines within a therapeutic group
which have such similar safety and efficacy that they can be interchanged without any
expected change in therapeutic outcomes.115,116 Reference priced medicines are fully
subsidised, while patients using more expensive medicines are required to pay part or all of
the extra cost.115,116 Schneeweiss et al. conducted a study in British Columbia, Canada to
determine the effects of the introduction of reference pricing for angiotensin converting
enzyme (ACE) inhibitors on the use of health care resources and costs (as a proxy for
health status), admission to aged care facilities and mortality for patients who switched
products.115 They included community dwelling patients aged over 65 who were taking the
ACE inhibitors that attracted a price premium after introduction of the policy.115 Data were
obtained from an administrative claims database and details of 37,000 patients were
analysed.
Over 14% of patients switched to a reference priced ACE inhibitor in the six months after
introduction of the policy, 75% stayed on the same ACE inhibitor, three percent stopped all
antihypertensives and eight percent switched from ACE inhibitor therapy to other
antihypertensives.115 There was an eleven percent increase in GP visits related to
cardiovascular conditions for patients who switched to benchmark priced ACE
inhibitors.115 This increase only lasted for two months. Three months after introduction of
the policy GP visit rates were the same for those who switched and those who didn’t.115
Although the increase in GP visits was associated with a transient increase in health care
costs, this was offset by the reduced cost of medication over time. There was no difference
in hospitalisation or mortality rates for those who switched ACE inhibitor and those who
didn’t.115 However, patients were only followed-up for six months post switching.
Worsened health outcomes (and associated increased health resource utilisation) may take
longer to manifest. Patients who switched were less likely to enter a nursing home than
non-switchers;115 however, this finding may have been associated with the fact that sicker
patients could obtain exemption from the reference pricing policy and were therefore
probably more likely to be non-switchers.
Overall, $6 million (Canadian) was saved in the first year post introduction of reference
pricing for ACE inhibitors through decreased medicine costs.115 Introduction of reference
pricing for ACE inhibitors in Canada resulted in some patients switching to cheaper
61
medicines, however, the switching did not result in increased health resource utilisation by
these patients.
A similarly designed study was conducted by the same authors following the introduction
of reference pricing for dihydropyridine calcium channel blockers in British Columbia.117
Switchers had a transient increase in GP visits for cardiovascular conditions in the months
post introduction of the policy, however, visits returned to baseline levels after three
months. Similarly, there was no difference in the rate of hospitalisation for switchers and
non-switchers.117 Expenditure on calcium channel blockers decreased by $1.62 million
(Canadian) in the year post introduction of the reference pricing policy.117
Other studies have also assessed the impact of introduction of reference pricing in Canada
on the utilisation of medicines in therapeutic groups including nitrates,118 and histamine-2
(H2) receptor antagonists.119,120 These studies did not assess the impact of introduction of
reference pricing on patient health outcomes. However, similar results were seen with
respect to medicine use and costs as were seen following introduction of reference pricing
for ACE inhibitors and calcium channel blockers. Overall dispensings for nitrates and H2-
receptor antagonists tended to remain stable after introduction of the reference pricing
policy, with a trend of decreased dispensings for premium priced medicines and increased
dispensings of co-payment priced products.118-120 Overall, cost savings for medicines in the
reference pricing therapeutic groups were achieved.118-120
When reference pricing was applied to statins in New Zealand, a study of patients who
switched from simvastatin to fluvastatin (the new reference priced product) found that
there was a significant increase in LDL, total cholesterol and triglyceride levels following
the medicine change.116 Two hundred and sixty two patients at a single hospital who
switched from simvastatin to fluvastatin following introduction of reference pricing were
studied to determine the impact of switching on health outcomes.116 Fasting lipid levels
from the last blood test prior to switching from simvastatin were compared with fasting
lipid levels taken up to six weeks after commencing fluvastatin. In over 90% of patients
who switched, there was a significant increase in LDL, total cholesterol and triglyceride
levels.116 Under-dosing and lack of potency of fluvastatin compared to simvastatin was
attributed to the lipid increases.116 However, meaningful interpretation of these results is
difficult, because a comparison group of patients who did not switch from simvastatin to
fluvastatin was not included. It is not possible to determine from this study whether the
changes in lipid control seen in switchers were actually associated with switching, or
whether it would have occurred anyway if the patients had continued using simvastatin.
62
3.3.2 Therapeutic group premium and reference pricing policies – some
conclusions
Results of Australian research have shown that the introduction of the therapeutic group
premium policy to the PBS and RPBS has generally led to increased use of benchmark
priced products and decreased use of premium priced products; and an overall reduction in
government expenditure on the therapeutic groups to which the therapeutic group premium
policy applies.112 However, overall use of medicines in the therapeutic groups affected by
therapeutic group premiums also decreased since introduction of the policy,112 suggesting
that some patients may have switched to medicines in other therapeutic groups or ceased
therapy altogether. The impact of the introduction of therapeutic group premiums on
patient compliance, medicine affordability and the associated health outcomes if patients
stop using their medicine have not been studied in Australia. However, appropriately
designed international studies suggest that switching to equivalent doses of reference
priced products does not have a negative effect on patient health outcomes.115,117 In
addition, international studies have shown that few patients cease therapy following the
introduction of reference pricing policies.115,117
3.4 PBS policies to influence supply and demand – overall
conclusions
The review of the literature in the previous sections highlighted the effects of increasing
patient co-payments, the minimum pricing and brand substitution policies, and the
therapeutic group premium policy on PBS and RPBS medicine dispensings. Potential
unintended effects of increasing patient co-payments were highlighted, including the
possibility that patients may go without their medicines or may under-dose due to the costs
associated with increasing co-payments.68-70 These findings were supported by
international research, which also found that patient health outcomes may be adversely
affected as a result of medicine cessation due to the purchase cost.71,72,74 The effect of large
PBS and RPBS co-payment increases in 2005 has not yet been assessed. However, it is
possible that decreased medicine use was associated with the co-payment increase and this
is an area requiring further investigation.
An analysis of the PBS therapeutic group premium policy found that in most cases, use of
co-payment priced medicines increased following introduction of the policy.112 However,
for the therapeutic groups affected by therapeutic group premiums, overall use of
medicines decreased. This suggests that some patients may have switched to other
medicines or ceased therapy altogether following introduction of the therapeutic group
63
premium policy. Research at the patient level has not been conducted, so the extent to
which introduction of therapeutic group premiums led to cessation of medicines is
unknown. Although evidence from international studies suggests that reference pricing
policies do not result in an increased rate of medicine cessation by patients,115,117 the
implications for Australian patients are unknown.
3.4.1 Gaps in the evidence base: the minimum pricing and brand
substitution policies and their potential influence on QUM
Increasing patient co-payments, brand premiums and therapeutic group premiums increase
the cost of medicines to patients, and the available evidence suggests that patients respond
to this price signal. There is the potential for quality use of medicines to be compromised if
patients cease use of medicines due to the increased cost. Although further research needs
to be conducted in the area of increasing co-payments and the therapeutic group premium
policy, in particular to investigate the effects of these policies on health outcomes for
patients, even larger gaps exist in the evidence base for the effect of the minimum pricing
and brand substitution policies on medicine dispensing and the quality use of medicines.
There has been only one published study on the effect of the minimum pricing and brand
substitution policies on PBS medicine dispensing. This study showed that patients
responded to the price signal associated with brand premiums and that use of co-payment
priced medicines increased following introduction of the minimum pricing policy.75 After
introduction of the brand substitution policy patients tended to switch to co-payment priced
medicines.75 International studies also showed that following introduction of generics,
patients tended to switch from the brand name product to cheaper generic
alternatives.33,94,95,98 Only two medicines were considered in the Australian research and
only the initial brand substitution was considered. The impact of the minimum pricing and
brand substitution policies on dispensing of other PBS subsidised medicines remains
unclear.
Concerns regarding potential unintended effects of brand substitution in Australia were
highlighted in numerous studies. Qualitative research conducted with consumers,9,80,81
pharmacists82 and prescribers88-90 highlighted concerns amongst these stakeholders that
patients may become confused by brand substitution. Prescribers voiced concern that
patients may have brand and generic products of their medicine substituted multiple times,
and that the potential for confusion may be worse with multiple brand substitutions.4-8,83-87
These concerns arise because, for many PBS medicines, there are multiple trade names for
products of the same medicine and these products frequently have different appearance.
64





Table 3.1 shows the different trade names for simvastatin, a medicine frequently dispensed
on the PBS and RPBS. Twelve different products were available in 2007, all of which had
different packaging. An example of the packaging for five of these products is shown in
Figure 3.1.
Table 3.1 - Multiple brand and generic products: different trade names for simvastatin
Chem mart simvastatin
Lipex
Simvahexal
Zimstat
GenRx simvastatin
Ransim
Simvar
Zocor
Genepharm simvastatin Simvabell
Simvasyn
Terry White Simvastatin
Figure 3.1 - Simvastatin packaging: appearance of different brand and generic products
A central theme of Australia’s National Medicines Policy is the “interdependence” of each
of the policy objectives.14 Interplay between access to medicines (via the PBS and RPBS)
and QUM is highlighted in the National Medicines Policy:
− “Effective mechanisms for access to medicines can assist quality use of
medicines, by reducing cost barriers to use of the most appropriate
treatment, but easy access can also work against quality use of
medicines.”14
Introduction of the brand substitution policy in 1994 reduced the “cost barrier” associated
with brand premiums. The brand substitution policy facilitates use of cheaper products, by
giving patients the opportunity to respond to the price signal associated with more
expensive brands of medicine. In this way, brand substitution may facilitate access to
medicines by allowing patients to avoid paying brand premiums. It may also facilitate
quality use of medicines by reducing the likelihood of under-use of medicines, which may
occur if patients cannot afford to pay for their medicines. However, there is the potential
for compromised quality use of medicines if patients have products substituted again
65
multiple times following the initial brand substitution.9 Despite widespread concerns that
multiple substitutions per patient occur,4-8,83-87 there is no published Australian research
regarding the number of brand substitutions per patient and the extent to which there are
multiple substitutions. Certain people have been identified as more vulnerable to confusion
from brand substitution than others, including the elderly, people with poor eyesight,
people with cognitive impairment and patients using multiple medicines.9,81 However, the
characteristics of people who are most likely to have brand and generic products
substituted and the characteristics of people who have multiple brand substitutions are
unknown. The situations in which multiple brand substitutions are most likely to occur are
also unknown. The need for evaluation of policies with the potential to influence quality
use of medicines has been noted;18 however, no prior Australian or international research
has addressed the rate or extent to which multiple brand substitutions occur. These gaps in
the evidence base represent the basis for the research.
The studies reported in this thesis explore the extent of brand substitution in selected
medicines, the extent of switching between brands by individuals and the characteristics of
patients for whom brands of medicine are substituted multiple times. In the next chapter,
the research aims are defined and the research designs which will be used for the studies in
this thesis are described.
66
CHAPTER 4
Research design
4.1 Introduction
The review of the literature in Chapter 3 highlighted the concerns of Australian
prescribers,88,89 pharmacists82 and consumers9,81 regarding the potential for patient
confusion when brand substitution occurs. It has been suggested that confusion may be
worse when products are substituted multiple times for a patient, and for patients using
multiple medicines, the elderly, people with vision impairment, those with cognitive
impairment, or people from non-English speaking backgrounds.4-9,12,83-85 However, the rate
and extent to which brand substitution occurs and the extent to which there are multiple
brand substitutions per patient are unknown. This research seeks to address these concerns.
In this chapter, the study designs and data source which will be used for the research are
described and an overview of the studies included in this thesis is provided.
4.2 Research aims
The overall goal of this research is to evaluate the impact of the minimum pricing policy
by examining the extent of brand substitution in Australia. This will be achieved by
exploring the frequency of brand substitution for selected medicines and the extent of
switching between brand and generic products by cohorts of individuals.
Specific aims of the research are to:
1. Determine the rate and extent of brand substitution of government subsidised
medicines in Australia, using seven examples: simvastatin, atenolol, citalopram,
enalapril, metformin, omeprazole and ramipril.
2. Determine the extent to which pharmacists substituted products which had not been
designated bioequivalent, using the example of simvastatin.
3. Identify the people who are at risk of having multiple brand substitutions.
67
4.3 Study design
In considering designs for this research, an experimental study design such as a
randomised controlled trial (RCT) was not possible because the minimum pricing policy
and brand substitution were implemented Australia wide. The policies apply to all patients
who receive medicines subsidised on the PBS and RPBS, so it is impossible to assign
patients to experimental “treatment” groups which are either exposed or unexposed to
brand premiums and brand substitution. In contrast, observational studies can evaluate
what happens in practice when patients are exposed to different treatments or
interventions.121 Researchers in observational studies have no control over whether patients
receive the treatment or exposure of interest; they simply observe what happens in real life
situations and compare outcomes between patients with and without the exposure of
interest.121
Many published studies assessing the impact of pharmaceutical policies on the use of
medicines, dispensing patterns and health resource utilisation have successfully used
observational study designs.75,112,115,117-120,122-131 Trends in dispensing of brand and generic
products following introduction of generics for fluoxetine and ranitidine were studied by
McManus et. al. using time series design.75 Changes in medicine dispensing on the PBS
and RPBS following co-payment changes68,112 and introduction of therapeutic group
premiums112 were also studied using time series designs. Cohort studies have been used to
determine the impact of reference pricing policies in Canada on medicine and health
resource use.115,117,120 Cohort designs have also been used to analyse the effects of
increases to patient co-payments on medicine dispensing patterns.123-131 The research in
this thesis will use time series and cohort designs.
To address the first aim of the research, time series designs will be used. Time series
designs involve repeat observations or measurements at regular intervals over time to
examine trends.132,133 Although brand substitution has been possible in Australia for over
ten years, the only published information regarding brand substitution trends for PBS
medicines considered fluoxetine and ranitidine over a three month period.75 Trends in the
rate of brand substitution over longer periods of time will be constructed for selected
government subsidised medicines.
Time series designs can provide evidence to support accepting or rejecting a research
hypothesis;134 in this case whether or not brand substitution occurs. However, because
patient level data are generally not used confounding from unmeasured variables may bias
68
results.134 This potential limitation will be overcome by conducting patient level analyses,
using cohort designs. Retrospective cohort studies will be conducted to identify the extent
of brand substitution per patient and to identify the characteristics of patients at risk of
having multiple brand substitutions. Cohort designs have been chosen because the
minimum pricing policy and brand substitution policy apply to all patients who receive
PBS and RPBS medicines. Cohort designs use entire populations (or subsets thereof),134
allowing inclusion of all people who receive medicines on the PBS and RPBS and who are
therefore subject to the minimum pricing policy and brand substitution.
In traditional cohort designs, patients are defined as being either exposed or unexposed to a
treatment or event of interest, and patients with different exposures may also be
compared.134 Patients are then followed in time, and differences in outcomes between
exposed and unexposed patients are compared.134,135 If exposed and unexposed patients in
the cohort are similar with respect to all factors which have the potential to influence the
outcome of interest, other than the exposure, then the rate of developing the outcome of
interest amongst unexposed people should represent the overall population rate for the
outcome of interest.135,136 If there are differences in the rate of developing the outcome of
interest between exposed and unexposed patients, then an association may be made
between the exposure and developing the outcome of interest.136 For the studies in this
thesis, different exposures will be compared for patients, with the exposure being the
extent of brand substitution for each patient (e.g. multiple brand substitutions compared to
few or no brand substitutions). Inclusion and exclusion criteria for each study are described
in detail in the relevant chapters.
Cohort studies are an appropriate study design to identify associations between an
exposure and patient outcomes when randomisation of patients to treatment groups is
unethical (e.g. when assessing the impact of exposure to a chemical on patient outcomes)
or not possible (e.g. when assessing the impact of introduction of pharmaceutical policies
which apply to entire populations, such as the minimum pricing policy and the brand
substitution policy).135 A disadvantage of all observational studies, including cohort
studies, is the potential for selection bias.134 This can arise if patients in the exposed and
unexposed groups have different baseline risks of developing the outcome of interest.137
Although statistical analysis can adjust for some differences between exposed and
unexposed patients (for example age, gender, socioeconomic status etc) if the groups differ
in some way which investigators have not considered or cannot measure, which influences
the outcome of interest, then bias will remain and cannot be controlled.137 However,
69
because entire populations (or subsets thereof) are included in cohort studies, the potential
for selection bias is much less than it is when other observational study designs are used.134
Another disadvantage of cohort studies is the requirement of large sample sizes,
particularly when rare outcomes are being studied.134 Prospectively conducted cohort
studies are expensive and the length of follow-up may be many years depending on the
outcomes of interest. Retrospective cohort studies using medical records or administrative
claims data can overcome these issues.134 Administrative health claims data from the
Department of Veterans Affairs (DVA) will be used for the studies in this thesis.
4.4 Study population and data source
Under the Veterans’ Entitlement Act 1986, the Australian Government Department of
Veterans’ Affairs (DVA) provides pharmaceutical, hospital, medical, dental and allied
health care to eligible veterans and their dependants.138 Administrative claims databases for
these health services are maintained by DVA139 and will be used for the present research.
These data were primarily chosen because the dataset has complete capture of all claims
for veterans and permission to access the data had been granted by DVA prior to
commencement of this research.
4.4.1 DVA treatment cards and level of entitlement to DVA subsidised health
services
DVA funded health services vary depending on a person’s level of entitlement. There are
three different DVA treatment cards with varying levels of entitlement – gold, white and
orange cards. DVA gold card holders are entitled to receive all medical, hospital,
pharmaceutical, dental and allied health care services funded by DVA.138 White card
holders are eligible to receive health services and pharmaceuticals subsidised by DVA for
the treatment of specified war or service related conditions only.138 DVA orange card
holders are eligible to receive pharmaceuticals subsidised on the RPBS, however they are
not entitled to DVA funded medical, hospital, dental or allied health care services.138
4.4.2 Characteristics of DVA treatment card holders
The DVA treatment population is approximately 290,000 people, comprised mainly of
Australian defence force veterans and their eligible dependants, such as widows or
widowers.140 A high proportion of the treatment population uses medicines (94%), with
42% using five or more regular medicines.141 Ninety two percent of DVA card holders
have poor eyesight requiring the use of corrective lenses.141
70
The DVA treatment population differs from the overall Australian population, particularly
with regards to age, gender and health resource utilisation. The DVA treatment population
is considerably older than the overall Australian population. Seventy eight percent of DVA
treatment card holders are aged over 65 years,140 compared to only 13% of the overall
Australian population.142 However there are 231,085 veterans aged 65 years or over140 and
the total number of Australians in this age group is estimated to be 2,668,648,142 meaning
that DVA treatment card holders represent 9% of older Australians.
Gender distribution of veterans also differs to the rest of the Australian population. Sixty
percent of all DVA card holders are male, and 52% of DVA card holders aged 65 years or
over are male.140 This is in contrast to the overall Australian population, where 50% of all
Australians and 45% of people aged 65 years or over are male.142
Age specific comparisons of DVA gold card holders with the wider Australian population
have shown that DVA gold card holders have slightly more GP visits (rate ratio 1.17; p <
0.05) and hospitalisations (rate ratio 1.21; p < 0.05) per year than other Australians.143
However, this appears to be associated with the level of service related disabilities among
veterans. DVA treatment card holders with no service related disability have a similar
number of GP visits (rate ratio 0.99, p > 0.5) and slightly fewer hospitalisations (rate ratio
0.97, p < 0.05) per year compared to other elderly Australians.143 The likelihood of
receiving a prescription at a GP visit is similar for the DVA population and the wider
Australian population; however, due to their slightly higher rate of GP visits, DVA gold
card holders receive slightly more government subsidised prescriptions per year than other
Australians (rate ratio 1.13; p < 0.05).143
4.4.3 DVA administrative claims data
DVA maintains administrative claims datasets for medicine dispensing, private hospital
admissions and medical and allied health consultations funded for eligible treatment card
holders. The different DVA treatment card types (gold, orange and white) and the varying
levels of entitlement mean that DVA administrative claims data relating to private hospital
admission and medical and allied health consultation are only complete for gold card
holders, because they receive all of these services fully funded by DVA. In contrast, the
administrative claims data for medicine dispensing is complete for the entire DVA
treatment population.
Alongside of the administrative health claims datasets, DVA maintains a client file. Patient
demographic information including gender, age, date of birth, date of death, postcode of
71
residence, DVA treatment card type and family status is included in the client file.144,145
Unique client identifiers are included in all of the DVA datasets, meaning that linkage
across datasets is possible.
4.4.3(i) Pharmacy data
When Pharmaceutical Benefits Scheme (PBS) and Repatriation Pharmaceutical Benefits
Scheme (RPBS) medicines are dispensed and the cost of the medicine is more than the
patient co-payment, pharmacists submit a claim to the government to obtain
reimbursement for the cost of the medicine.146,147 Information relating to all medicines
dispensed to eligible veterans subsidised by DVA on the PBS and RPBS is stored in the
DVA pharmacy claims dataset. Currently the dataset contains approximately 75 million
dispensing records.144,145 Information recorded for each claim includes the medicine
dispensed, the strength, dosage form, brand and quantity dispensed, the RPBS eligibility
status, the date of prescription and supply, and whether an original or repeat prescription
was dispensed. Patient, dispensing pharmacy and prescriber identifiers are also included.
The RPBS co-payment was $5.00 in 2008,23 and because all dispensed prescriptions cost
more than this amount, all medicines dispensed to DVA treatment card holders receive a
government subsidy and appear in the pharmacy claims database.
4.4.3(ii) Private hospital admissions data
DVA funds private hospital admissions for eligible treatment card holders. Within the
hospital dataset, information relating to the date of hospital admission and discharge is
stored. Primary and secondary diagnoses for each hospital admission and information
relating to procedures performed during the admission are recorded. Only private hospital
admissions are captured within the DVA administrative claims dataset. This represents
60% of all hospital admissions for DVA card holders.141
4.4.3(iii) Medical and allied health data
The DVA medical and allied health dataset provides information relating to the type of
DVA funded health service received by a patient (e.g. all Medicare listed services, and
allied health services funded by DVA including physiotherapist and podiatry consultations)
and the date on which the consultation occurred. Information relating to whether a DVA
treatment card holder is a resident of an aged-care facility is included, as is the date of
admission to the facility and the level of care (e.g. high or low) required.
72
4.4.4 Appropriateness and relevance of DVA administrative claims data for
use in the present research
The completeness of the DVA claims data and the ability to link pharmacy, private
hospital, medical and allied health datasets make them appropriate for use in the present
research. An advantage of DVA data is complete coverage for patients. After a patient has
qualified for a DVA treatment card, they cannot become ineligible for coverage at a later
date. The characteristics of veterans also make them relevant candidates for studying the
research aims. They represent those likely to be at risk of confusion from multiple brand
substitutions, such as the elderly, people using multiple medicines and people with poor
eyesight.9 Patient identifiers allow identification of medicine dispensings (and therefore
brand substitution) at the patient level. The suitability of the information contained within
DVA datasets and the relevance of the DVA treatment population for studying the research
aims makes DVA data an appropriate source to be used for the present research. A
complete list of the data available, relevant to the present research, is listed in Appendix
4.1.
4.5 Preview of studies included in this thesis
The first study in this thesis, reported in Chapter 5, aims to determine trends in the rate of
brand substitution using the example of simvastatin, a medicine for which brand
substitution has recently become possible. The study will examine trends in the overall rate
of brand substitution in patients who received simvastatin in the sixteen months after
generics became available. This will be compared with the dispensing trend prior to
generic simvastatin availability to gain a picture of the change (if any) in the overall rate of
substitution when generics first became available. A patient level analysis will also be
undertaken for simvastatin to identify the extent of brand substitution per patient following
introduction of generics for the first time.
To build on the evidence from the study of simvastatin in Chapter 5, Chapter 6 presents a
study examining trends in the rate and extent of brand substitution for six additional
government subsidised medicines. The study is reported in three parts. In Part 1, trends in
brand substitution rates for the six study medicines are presented. Having determined
overall trends in brand substitution rates, Part 2 of Chapter 6 examines the extent of brand
substitution within a single prescription form (the original supply plus five repeat
dispensings) for the six study medicines to address concerns that there is the potential for
patients to receive a different brand or generic product each time their prescription repeats
are dispensed. Having ascertained the extent of brand substitution “per prescription”, the
73
third part of the study examines the extent of brand substitution “per patient” for the total
duration of use (i.e. until cessation or death) using a retrospective cohort design. The
association of patient age, gender, number of prescribers and number of dispensing
pharmacies on the number of brand substitutions per patient is examined.
The study in Chapter 6 considers brand changes among individual medicines; however, on
average, elderly Australians use at least four prescription medicines148 and 42% of DVA
treatment card holders use five or more regular medicines.141 To address concerns that
patients may have multiple switches for many of their medicines,4,7,84,87,149 in Chapter 7 a
retrospective cohort study is presented examining the extent of brand substitution for all of
the medicines used by a patient. To identify factors associated with increased risk of
having multiple brand substitutions, the effect of patient age, gender, total number of
prescription medicines, number of prescribers, number of dispensing pharmacies, number
of hospital admissions, socioeconomic status, location of residence (city vs. country, and
living in the community vs. a residential aged care facility), having a home medicines
review and number of co-morbidities is assessed.
The aims and methods used for each of these studies, and the relevant data extracted from
the DVA administrative claims databases are described in each chapter.
4.6 Ethical approval
Prior to commencing the research, ethical approval was obtained from the Department of
Veterans’ Affairs Human Research Ethics Committee.
74
CHAPTER 5
Brand substitution of government subsidised medicines
in Australia: the example of simvastatin
5.1 Introduction
As highlighted in Chapter 2, brand substitution of Pharmaceutical Benefits Scheme (PBS)
and Repatriation Pharmaceutical Benefits Scheme (RPBS) medicines can occur if the
patient agrees to the product change, if the substituted products are identified as
bioequivalent in the Schedule of Pharmaceutical Benefits and if the prescriber has not
precluded brand substitution from occurring.2 The cheapest brand or generic product(s) of
each medicine are available to patients for the PBS co-payment, while more expensive
products attract a brand premium that patients are required to pay in addition to the co-
payment. Although this legislation has been in place since December 1994, the rate and
extent of brand substitution in Australia has not been extensively studied. In this chapter
trends in the rate and extent of brand substitution, using the example of simvastatin, are
reported.
Simvastatin is a HMG-CoA reductase inhibitor, marketed in Australia for the treatment of
hypercholesterolaemia. Prior to November 2004 two brand name simvastatin products
were available; however bioequivalence had not been reported,150 so brand substitution
should not have occurred. Patients could only receive different products at consecutive
dispensings if they had more than one prescription form with different brands prescribed.
The first simvastatin generic was PBS listed on November 1st 2004 and at that time
bioequivalence was shown for the new generic and the two original brands, so brand
substitution was possible. A second generic was PBS listed in April 2005,151 and by the
end of August 2005 ten bioequivalent products were available.152 This is summarised in
Table 5.1.
75
Table 5.1 - Simvastatin: timeline of PBS/RPBS listing of brand and generic products
Simvastatin – timeline of PBS/RPBS listing
Pre-brand substitution
Simvastatin PBS/RPBS listed
1990
policy, so brand
Two products available
substitution not possible
Brand substitution policy introduced
December
Brand substitution of
Two simvastatin products available
1994
simvastatin not possible
Bioequivalence not indicated
First simvastatin generic PBS/RPBS listed
November
Bioequivalence indicated for the two
Brand substitution of
2004
original products and the new generic
simvastatin possible
i.e. Three substitutable products
Second simvastatin generic PBS/RPBS listed
April
Bioequivalence indicated for the two
Brand substitution of
2005
original products and the new generic
simvastatin possible
i.e. Four substitutable products
Multiple simvastatin generics PBS/RPBS
listed
August
Brand substitution of
Ten substitutable products available
2005
simvastatin possible
Bioequivalence indicated for all
available products
The introduction of generic simvastatin products to the Australian market provides a
unique opportunity to study brand substitution because, within a short period of time,
multiple bioequivalent products were available for substitution. In addition, because two
non-substitutable products were originally available it provides an opportunity to examine
the extent to which pharmacists follow brand substitution guidelines. A high rate of
substitution prior to generic availability would suggest non-adherence by pharmacists to
the brand substitution policy guidelines.
Although brand substitution of PBS and RPBS medicines has been possible for over ten
years, little is known about the rate and extent of brand substitution when new generics are
listed for a medicine. The rate at which products were substituted prior to demonstration of
bioequivalence is also unknown.
76
5.2 Aims
This study aimed to identify the rate of substitution between brand and generic products of
simvastatin.
Secondary objectives of the study were to:
•
Determine the rate at which products were substituted prior to demonstration of
bioequivalence.
•
Determine if there are differences in the rate of switching for new simvastatin users
compared to stable simvastatin users who regularly received the same brand prior to
introduction of generics.
•
Identify the number of brand substitutions per patient.
•
Identify the association of the number of prescribers, number of dispensing pharmacies,
number of original prescriptions used, and number of simvastatin dispensings on the
number of brand substitutions per patient.
•
Determine the frequency with which brand substitution occurs with use of a new
prescription compared to having repeats of the same prescription dispensed
consecutively.
•
Identify the number of different brand and generic products dispensed over the life of
individual prescription forms.
5.3 Methods
The study was conducted using DVA pharmacy claims data for simvastatin. Claims
dispensed between November 1st 2002 and February 28th 2006 were included in the
analysis. Information extracted from the claim records for each dispensing included a
patient identifier, the date of dispensing, strength of simvastatin dispensed, the
manufacturer code (indicating the brand or generic product supplied), a prescriber
identifier, the date of prescription, a dispensing pharmacy identifier, whether an original
prescription or a repeat was dispensed and the total number of repeats ordered for that
prescription.
5.3.1 Identification of switches
The term “switch” was chosen to identify situations when different brand or generic
products were supplied at consecutive dispensings. To avoid identification of brand
changes when patients ceased therapy and then recommenced at a later date using a
77
different product, a time limit was calculated from the simvastatin claims included in this
study for use in the identification of switches.
The most common time interval between consecutive simvastatin dispensings for the
claims included in this study was 28 days (Table 5.2). This reflects the PBS and RPBS
subsidised quantity of simvastatin which is thirty tablets, equating to approximately one
months supply at standard doses. Ninety five percent of consecutive dispensings for
simvastatin occurred within sixty days, which is approximately twice the expected duration
of supply for the PBS and RPBS subsidised quantity of simvastatin. It allows for patient
non-compliance without being a wide enough time interval to capture situations when
patients cease therapy and then re-start at a later date.
Therefore, switches were identified when different brand or generic products were supplied
at consecutive dispensings within sixty days.
Table 5.2 - Simvastatin: time between consecutive dispensings
Time between
dispensings (days)
Mean ± SD:
32.7 ± 16.4
Median:
30.0
Mode:
28
Range:
0 - 177
Percentiles:
25th 26
75th 36
90th 48
95th 59
96th 63
The manufacturer code was the variable used to identify the brand or generic product
dispensed. If the manufacturer code was not recorded for a claim, it was assumed that the
same product was dispensed consecutively. Analysis of the 1.4 million claims identified
for this study showed that the manufacturer code was missing for only 4%.
The rate of switching at consecutive dispensings was calculated per one thousand
prescriptions dispensed each month.
Patients who did not receive simvastatin in the year prior to generic availability (between
November 1st 2003 and October 31st 2004) were defined “naïve” users; while patients who
received at least three dispensings of the same simvastatin product in the four months prior
78
to generic availability (between July 1st and October 31st 2004) were defined “stable”
users. The rate of switching after introduction of simvastatin generics was calculated for
naïve and stable users.
The total number of switches per patient during follow-up was identified. Patients were
categorised as non-switchers, those with only one or two switches and those with three or
more switches post-generics (“multiple switchers”) for sub-group analyses.
To determine if there is an association between number of prescribers and dispensing
pharmacies and the number of switches per patient, the number of simvastatin prescribers
and dispensing pharmacies was identified for each patient from the claims data.
To determine whether use of new prescriptions was associated with switching, it was
determined whether the same or a different prescription was used at consecutive
dispensings when switches occurred. The prescription was identified by the date of
prescription and the prescriber identifier. Doctors can write PBS and RPBS prescriptions
for simvastatin valid for up to six supplies (the original prescription plus a maximum of
five repeat dispensings); and can only write one original prescription for each simvastatin
strength per patient per day.150 Therefore it was considered that a patient had repeats of the
same prescription dispensed consecutively if consecutive claims showed the same
prescriber identifier and date of prescribing, and were for the same strength of simvastatin.
Original and repeat dispensings for individual prescriptions were identified from claims
with the same prescriber identifier and date of prescription. The total number of
dispensings per prescription was calculated. The simvastatin product supplied at the
original and each repeat dispensing was identified for prescriptions written for five repeats
with all six supplies dispensed between November 1st 2004 and February 28th 2006. The
number of different brand or generic products supplied over the life of these prescription
forms was calculated.
5.3.2 Statistical analysis
Overall switching rates pre- and post-generic availability were compared using negative
binomial regression. Differences in switching rates between stable and naïve users were
analysed using a negative binomial regression, with an offset for subject length of follow-
up. Post hoc pair wise comparisons were undertaken to compare the rate of switching for
stable and naïve users at different time periods. Differences in the number of simvastatin
prescribers, dispensing pharmacies, dispensings and number of prescriptions used were
compared between non-switchers, patients with only one or two switches and multiple
79
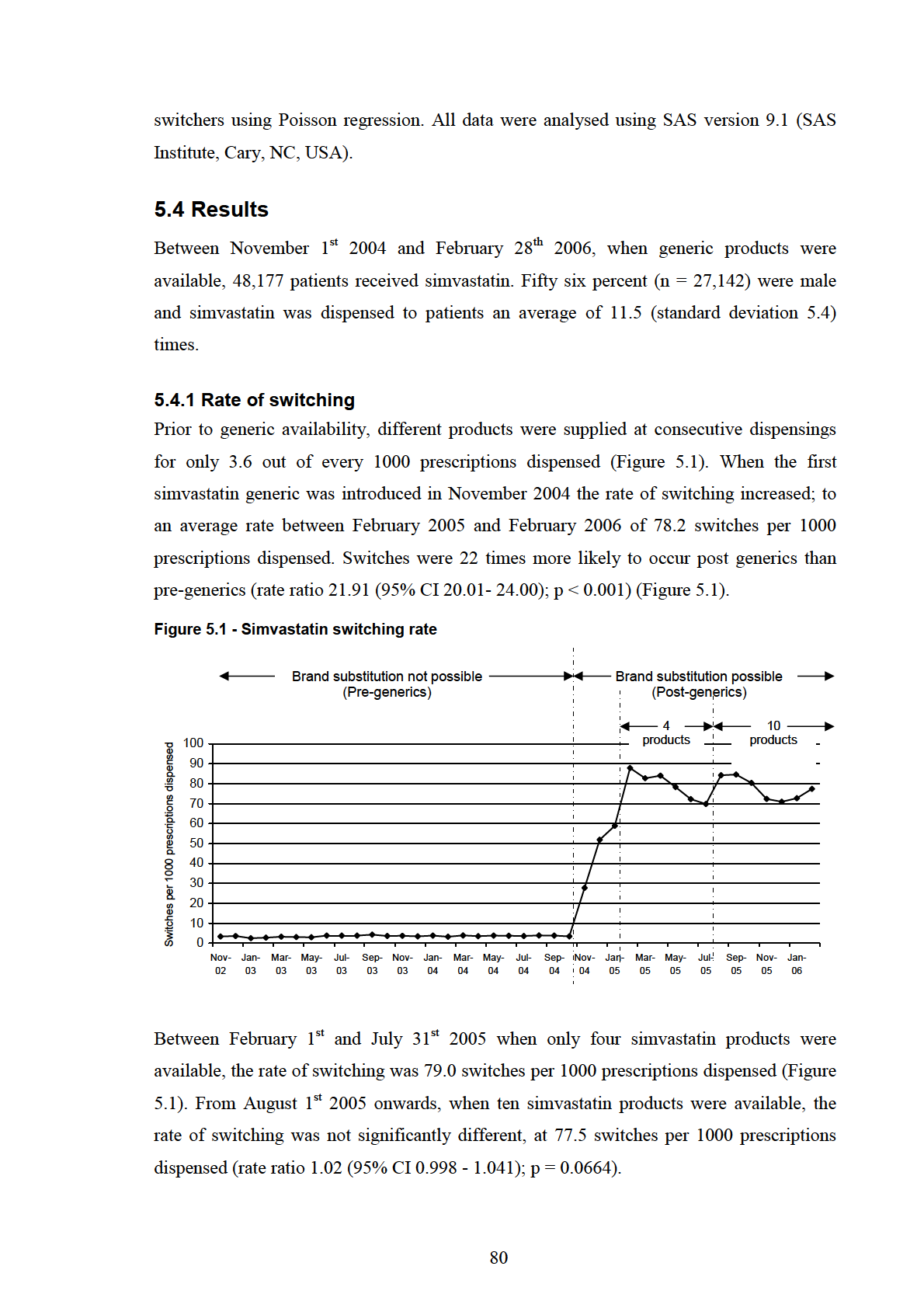
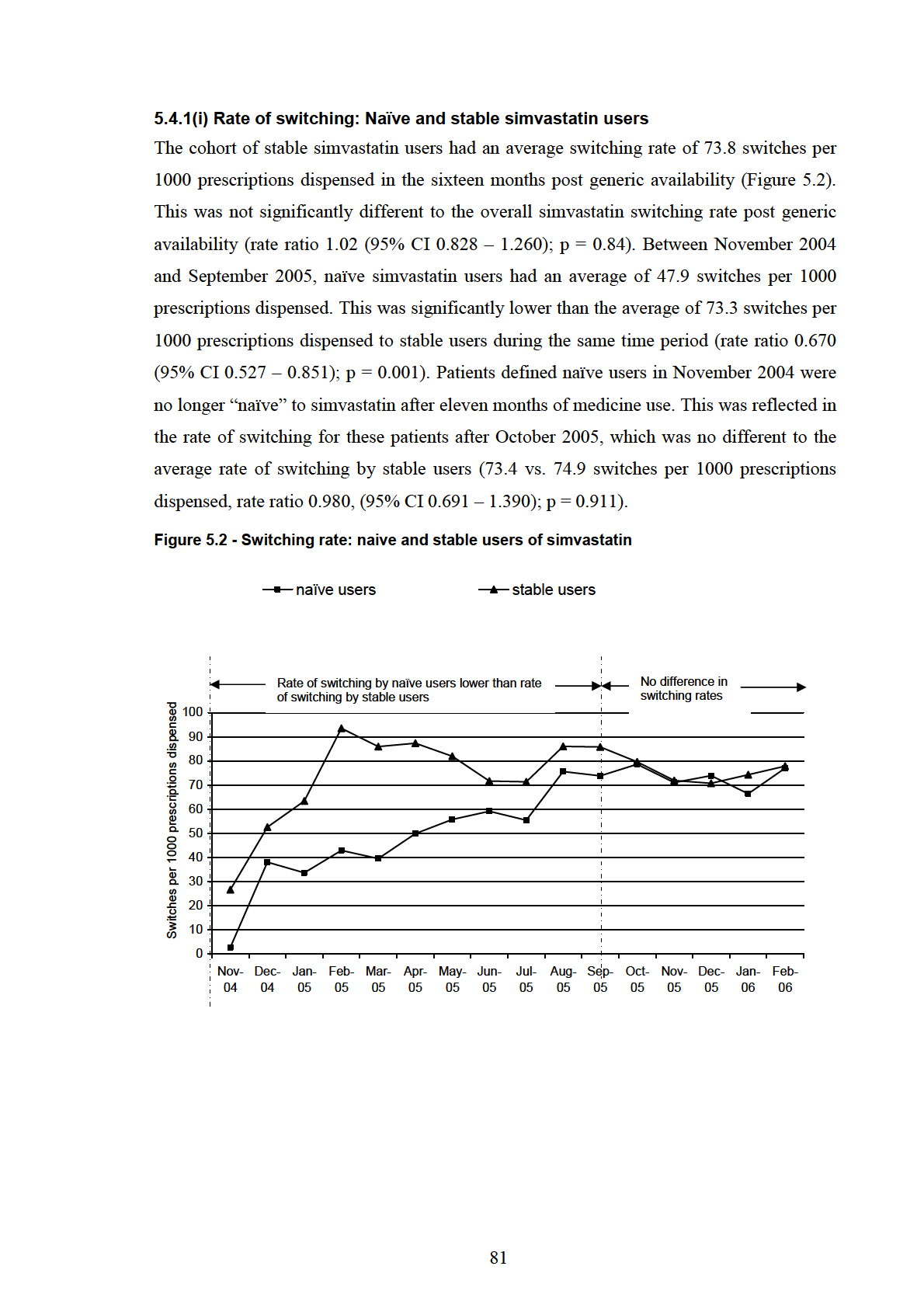
5.4.2 Patients with and without switches
Fifty three percent of patients who received simvastatin post-generic availability did not
switch products. Thirty eight percent of patients had only one or two brand substitutions,
while 9% had multiple switches. Poisson regression showed that multiple switchers were
significantly more likely to have more prescribers, more dispensing pharmacies, more
original prescriptions and more simvastatin dispensings than non-switchers (Table 5.3) and
patients who had only one or two switches (Table 5.4).
Table 5.3 - Patients dispensed simvastatin: factors associated with being a multiple
switcher compared to a non-switcher
Multiple switchers
Non-switchers
Rate ratio (95% CI)*
n = 4,205
n = 25,633
Mean number of:
(± standard deviation)
Prescribers
1.93 ± 1.07
1.37 ± 0.63
1.41 (1.38 – 1.45)
Dispensing
2.56 ± 1.61
1.44 ± 0.88
1.77 (1.73 – 1.81)
pharmacies
Original
4.54 ± 1.65
2.84 ± 1.46
1.60 (1.58 – 1.63)
prescriptions
Dispensings
15.17 ± 3.09
9.48 ± 5.84
1.60 (1.59 – 1.61)
*p < 0.0001 for all comparisons
Table 5.4 - Patients dispensed simvastatin: factors associated with having multiple switches
compared to one or two switches
Multiple switchers
Patients with one
Rate ratio (95% CI)*
n = 4,205
or two switches
n = 18,339
Mean number of:
(± standard deviation)
Prescribers
1.93 ± 1.07
1.61 ± 0.80
1.20 (1.17 – 1.23)
Dispensing
2.56 ± 1.61
1.69 ± 1.04
1.51 (1.48 – 1.54)
pharmacies
Original
4.54 ± 1.65
3.74 ± 1.24
1.22 (1.20 – 1.24)
prescriptions
Dispensings
15.17 ± 3.09
13.50 ± 3.81
1.12 (1.11 – 1.13)
*p < 0.0001 for all comparisons
82
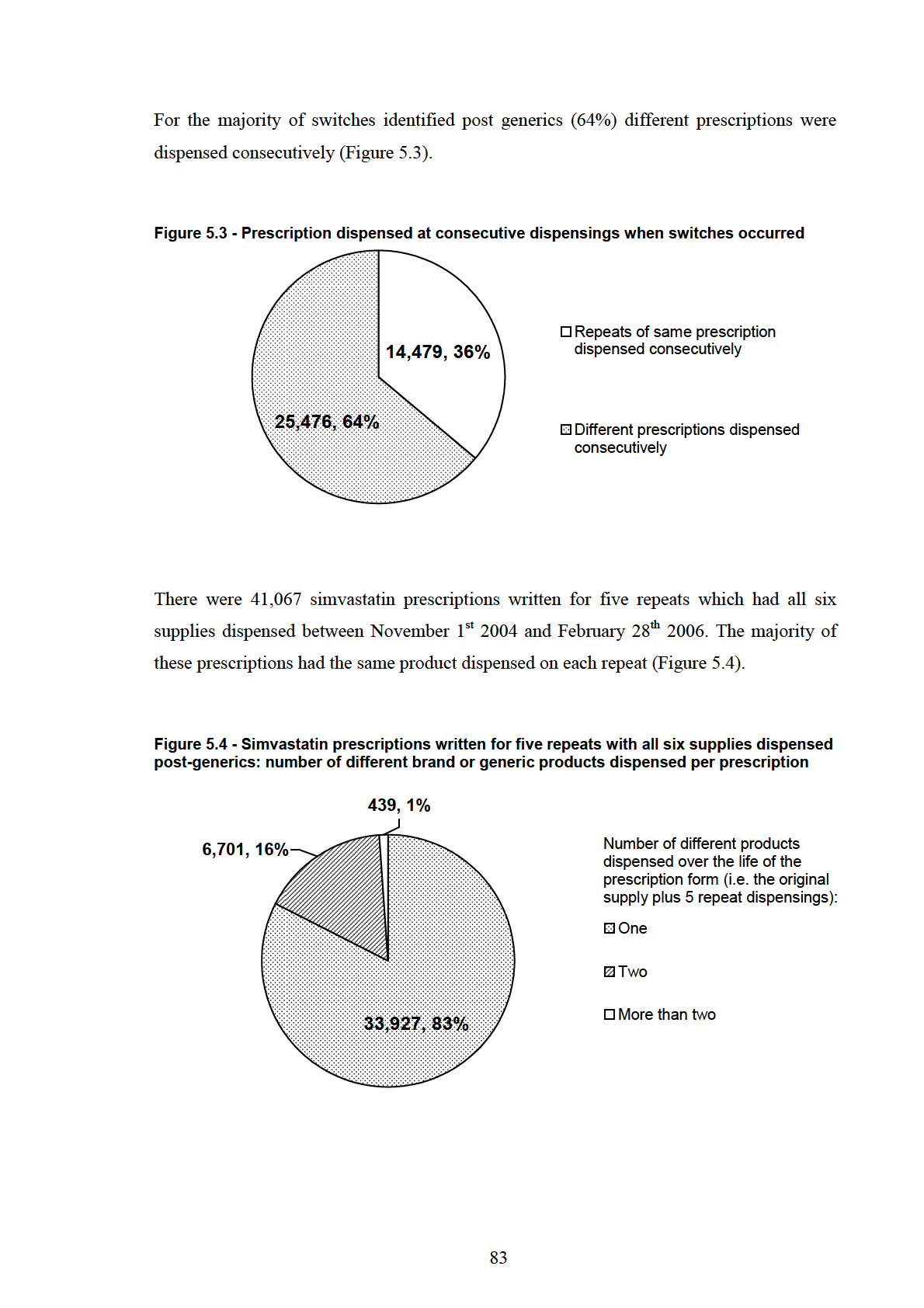
5.5 Discussion
Results of this study suggest that, in the case of simvastatin, the brand substitution policy is
being implemented primarily as intended. While nearly half of the patients who received
simvastatin post-generic availability switched products, the majority of patients had only
one or two brand substitutions. Switching was 22 times more likely to occur post-generic
availability compared to pre-generics; however, the increase was largely due to patients
switching once to a new generic product, rather than patients being switched multiple
times. In the sixteen month study period, 91% of patients switched twice or less. These
results add to previous Australian research where it was shown that in a three month period
after introduction of brand premiums to ranitidine and fluoxetine, 8% and 39% of patients,
respectively, switched to cheaper products.75 The results of the present study demonstrate
that brand substitution is sustained for longer periods after generics are introduced.
In the first eleven months after generic simvastatin became available, stable simvastatin
users had a higher rate of switching than did naïve users. The product dispensed to stable
users prior to introduction of the first generic attracted a brand premium after November 1st
2004. It is likely that many stable users switched to one of the new generics to avoid
paying the brand premium. However, the association between the product initially received
by a patient (e.g. co-payment priced compared to a product with a brand premium) and the
number of switches during follow-up was not considered for this study. This will be
studied in later chapters of this thesis. Patients who were naïve users of simvastatin in
November 2004 were no longer naïve to the medicine after eleven months of use. This was
reflected in the switching rate after October 2005, which was no different to the rate of
switching by stable users.
It has been suggested that some pharmacies frequently change the generic product(s) that
they stock, which has the potential to result in patients receiving a different generic each
time the stocked generic product changes.5,8,83 The large number of products available for
some medicines has been suggested as a potential source of patient confusion if brand
substitution occurs frequently.5,8 In this study, the overall rate of switching was no
different when ten simvastatin products were available for substitution compared to when
only four products were available. Availability of multiple simvastatin products was not
associated with an increased rate of switching in this study.
A small proportion of patients in this study (9%) had multiple switches. Results of
univariate analysis showed that multiple switchers were more likely to have multiple
84
prescribers, dispensing pharmacies and prescriptions, suggesting that continuity of care
may be associated with multiple brand substitutions. Use of new prescriptions may also
play a large role, as different prescriptions were dispensed at consecutive dispensings for
the majority of switches identified (64%). When individual prescriptions were considered,
the majority had the same product dispensed on each repeat. The product supplied to a
patient at the previous dispensing may not be apparent to the dispensing pharmacist if a
different prescription was used, or if the patient attended a different pharmacy. Results of
the univariate analysis provided an indication of the situations in which multiple switches
may occur. This will be investigated further in studies reported in later chapters of this
thesis using multivariate statistical methods.
The results of this study suggest that pharmacists adhere to the rules of the brand
substitution policy by rarely substituting products that have not been designated
bioequivalent. Prior to introduction of the first simvastatin generic, different products were
supplied at consecutive dispensings for only 3.6 out of every 1000 dispensings. A high
switching rate prior to generic availability may have suggested that pharmacists substituted
products which hadn’t been shown to be bioequivalent. The low pre-generics switching
rate suggests that substitution is likely to have occurred only in situations where it was
permitted (e.g. when the patients usual product was out of stock), or when patients had
more than one prescription, written for different brands, dispensed consecutively. The
change of legislation allowing brand substitution occurred in 1994 and more than ten years
later, it appears pharmacists adhere to this legislation.
Findings of this study are strengthened by the long run-in period prior to introduction of
generics, and the extended follow-up post-generics. Data were available for two years prior
to introduction of simvastatin generics, and the rate of switching was consistently low
during this two year period.
This study only considered the extent of brand substitution for one medicine for which
brand substitution had recently become possible. The rate of switching between brand and
generic products for other medicines, including those for which brand substitution has been
possible for longer periods of time, remains unclear. In addition, the rate of switching and
the number of switches per patient may have been underestimated by the assumption that
the same product was supplied at consecutive dispensings when the manufacturer code was
missing for a claim. It is possible that in some cases different products were supplied at
consecutive dispensings when manufacturer codes were not recorded. However, this is
85
unlikely to have influenced the results to a large degree, because only 4% of included
claims had the manufacturer code missing.
5.6 Conclusions
The results of this study showed that brand substitution was more common following
introduction of simvastatin generics; however the increase was due to the large proportion
of patients who substituted to a new generic product. The majority of patients did not have
products substituted and only a small proportion of patients, 9%, had multiple brand
substitutions. The results of this study suggest that pharmacists adhere to brand substitution
legislation in the case of simvastatin by rarely substituting products for which
bioequivalence has not been demonstrated. In the case of simvastatin, the brand
substitution policy appears to be implemented primarily as intended. From a quality use of
medicines perspective, pharmacists appear to be adopting an approach that minimises the
likelihood of patient confusion which has been hypothesised to arise from multiple
switching of brands.
It is unclear whether this is also the case for other government subsidised medicines. In the
following chapter of this thesis, trends in the rate and extent of brand substitution for six
other government subsidised medicines are examined. The extent of brand substitution
“per prescription” and “per patient” will be studied for these six case examples. Situations
in which multiple brand substitutions occur will be identified.
86
CHAPTER 6
Analysis of the extent of brand switching for six
medicines: atenolol, citalopram, enalapril, metformin,
omeprazole and ramipril
6.1 Introduction
The study of simvastatin in Chapter 5 confirmed that following introduction of
bioequivalent simvastatin products, a large increase in brand substitution occurred. Only a
small proportion of patients however had multiple switches. In this chapter the extent of
brand switching for six other government subsidised medicines will be studied to further
understand the manner in which brand substitution occurs in Australia. The study in this
chapter is presented in three parts.
In Part 1 a background analysis is presented, analysing trends in the rate of brand
substitution for the six study medicines: atenolol, citalopram, enalapril, metformin,
omeprazole and ramipril. This analysis is undertaken to confirm that brand substitution
occurs for each of the medicines and that further study of these six medicines is relevant.
Part 2 of the study presents the extent of brand substitution on repeats of individual
prescription forms. On the PBS and RPBS, prescriptions are valid for one year from the
date of prescription2 and the standard dispensed quantity of medicine usually equates to
one months supply. When continuing therapy is required the prescriber may order the
original one months supply and up to five one month repeat supplies; equating to
approximately six months supply of medicine at standard doses per prescription form. In
2003, the Australian Divisions of General Practice (ADGP) expressed concerns about the
potential for patients to receive a different brand of medicine each time the original
prescription and repeats were dispensed, equating to six different brands dispensed over
the life of an individual prescription form.4 ADGP suggested that a formal limit of one
switch per prescription should be enforced.4 At present, the rules of the brand substitution
87
policy do not specify a limit to the number of switches that can occur on the repeats of an
individual prescription, so there is the potential for multiple switches to occur. Part 2 of
this chapter aims to identify the extent of brand substitution per prescription using this
“prescription-level” analysis for the six study medicines.
Having established the extent of switching per prescription, Part 3 of this study goes on to
examine the number of brand switches per patient for the total duration of medicine use
(until therapy cessation or patient death); i.e. a “patient-level” analysis. The
Pharmaceutical Society of Australia (PSA) has guidelines for brand substitution by
pharmacists, which recommend the consistent supply of the same brand or generic product
to chronic users of medicine.153 Part 3 of this study examines whether pharmacists are
adhering to the PSA brand substitution guidelines.
6.2 Aims
The overall aim of the study reported in this chapter was to identify the extent of brand
substitution for the six study medicines.
The specific objective of Part 1 of the study was to identify trends in the rate of brand
switching for the six study medicines.
Objectives of Part 2 were to:
•
Identify the number of brand switches on the repeats of an individual prescription; a
“prescription-level” analysis.
•
Determine how often multiple products are supplied on the same prescription.
The objectives of Part 3 of the study were to:
•
Identify the number of brand switches per patient; a “patient-level” analysis.
•
Identify the association of the number of prescribers, number of dispensing pharmacies,
duration of follow-up and patient age with the number of brand switches per patient.
•
Determine if there are differences in the number of brand switches for patients initiated
on co-payment priced products compared to patients initiated on products with a brand
premium.
88
6.3 Methods
Atenolol, citalopram, enalapril, metformin, omeprazole and ramipril were selected as study
medicines. These medicines were chosen because they represent a range of therapeutic
classes (Table 6.1), they are all commonly dispensed on the PBS and RPBS52 and are
usually used in the treatment of chronic conditions. All of these medicines have
bioequivalent brand and generic alternatives available. Brand substitution has been
possible for the six medicines for varying lengths of time. Analysis was limited to the
strengths and formulations of these medicines with substitutable brand and generic
products listed in Table 6.1.
Table 6.1 - Study medicines
Study medicine
Strengths
Number
Therapeutic category
Brand
and forms
of
and uses
substitution
studied
products
possible since:
Atenolol
50mg tablets
10*
Beta blocking agent used in
1994
the treatment of
cardiovascular conditions
including hypertension
Citalopram
20mg tablets
7
SSRI antidepressant
August 2001
Enalapril
5mg, 10mg
11*
Angiotensin converting
May 2000
and 20mg
enzyme inhibitor used in the
tablets
treatment of cardiovascular
conditions including
hypertension
Metformin
500mg and
10*
Biguanide oral
1996
850mg
hypoglycaemic agent, used in
tablets
the treatment of type 2
diabetes
Omeprazole
20mg tablets
2 (3 from Proton pump inhibitor used in
1999
Dec 2004 the treatment of acid related
onwards) gastrointestinal disorders
such as gastroesophageal
reflux disease and peptic
ulcer disease.
Ramipril
1.25mg,
2
Angiotensin converting
1998
2.5mg and
enzyme inhibitor used in the
5mg tablets
treatment of cardiovascular
conditions including
hypertension
*The number of products available for the majority of the time between 2001 and 2005 is
shown because single brand or generic products were added/deleted during this time period
89
DVA pharmacy claims for dispensings of the six study medicines between January 1st
2001 and February 28th 2006 were identified. Information extracted from each claim record
included a patient identifier, the date of supply, date of prescription, a prescriber identifier,
a dispensing pharmacy identifier, the manufacturer code (indicating the brand or generic
product dispensed), the number of repeats ordered for the prescription, whether the original
prescription or a repeat was dispensed and the number of times repeats of the prescription
had already been dispensed.
Analysis of citalopram was limited to dispensings after August 1st 2001, because brand
substitution was not possible for citalopram prior to this date. For all other study
medicines, brand substitution was possible for the entire study period so all claims were
eligible for inclusion.
6.3.1 Methods: Part 1 – Background analysis
Using the definition developed in Chapter 5 (section 5.3.1), switches were identified if
different brand or generic products of the same strength of medicine were supplied at
consecutive dispensings within sixty days. If the manufacturer code was not recorded for a
claim, it was assumed that the same product was dispensed consecutively.
The sixty day interval was calculated from the data used for this study and represents the
90th percentile for time between consecutive dispensings for the study medicines, and
approximately twice the expected duration of supply for the PBS subsidised quantity
(Table 6.2). The PBS and RPBS subsidised quantity for each of the study medicines
equates to approximately thirty days supply at usual doses. This is reflected in the most
common length of time between consecutive dispensings for patients who received each of
the medicines, which was 28 days.
90
Table 6.2 - Time between consecutive dispensings for the study medicines
Time between consecutive dispensings (days)
Atenolol Citalopram Enalapril Metformin Omeprazole Ramipril
Mean ±
standard
32 ± 14
29 ± 14
31 ± 20
33 ± 19
29 ± 13
31 ± 21
deviation:
Median:
30
28
29
29
28
29
Mode:
28
28
28
28
28
28
Range:
0 - 198
0 - 200
0 - 994
0 - 990
0 - 200
0 - 987
Percentiles:
25th
25
21
24
21
22
24
75th
36
34
35
41
35
35
90th
53
45
44
56
44
45
95th
62
56
56
64
54
56
96th
64
58
58
67
57
59
Major changes to the PBS listing of each of the study medicines during the study period
with the potential to influence the rate of switching were identified from the Schedule of
Pharmaceutical Benefits. The Schedule of Pharmaceutical Benefits lists all medicines
eligible for PBS subsidy, the brand and generic products available, the magnitude of any
applicable brand premiums, and indicates products which are bioequivalent. Changes with
the potential to influence the rate of switching were considered to be additions or deletions
of multiple bioequivalent products and addition of brand premiums to products previously
available at the co-payment price.
The rate of switching at consecutive dispensings was calculated per one thousand
prescriptions dispensed each month for each of the study medicines. Average switching
rates over the five years of follow-up were calculated.
6.3.2 Methods: Part 2 - The extent of brand switching on repeats of individual
prescriptions; a “prescription-level” analysis
In Part 2 of the study, the number of switches within a single prescription form (the
original supply plus five repeat dispensings) was studied; a “prescription-level” analysis.
6.3.2(i) Identification of dispensings for individual prescriptions
Doctors can write PBS and RPBS prescriptions for each of the study medicines valid for
up to six supplies (the original dispensing, plus a maximum of five repeat dispensings);
and can only write one PBS or RPBS prescription for each strength of study medicine per
91
patient per day.2 Following these rules it was determined that when claim records showed
the same patient identifier, prescriber identifier, date of prescription and were for the same
strength of medicine, repeats of the same prescription had been used at those dispensings.
Individual prescriptions were identified and original and repeat dispensings were sorted by
date of supply. The total number of dispensings per prescription was calculated.
Data errors were identified if there was more than one claim for an original prescription
written by the same doctor on the same day for any patient, or if there were more claims
identified for a prescription than allowed by the number of repeats ordered. For example, if
a prescription had five repeats ordered but more than six claims for dispensing of that
prescription appeared in the claims data, a data error was identified. All claims for these
prescriptions were excluded. This led to 16,175 dispensings excluded (0.3% of identified
dispensings).
Prescriptions written for fewer than five repeats, and prescriptions written for five repeats
but which did not have all six supplies dispensed within the study period, were excluded
from analysis in this part of the study.
6.3.2(ii) Identification of switches for the prescription-level analysis
The manufacturer code in each claim record was used to identify the brand or generic
product dispensed. It was assumed that the original dispensing was for the brand or generic
product written on the prescription; switches were then identified if different brand or
generic products were supplied on consecutive repeat dispensings of the same prescription.
When the manufacturer code was not recorded for a given dispensing (3% of claims
included in Part 2), it was assumed that it was the same as the previous supply. The
number of switches per prescription and the number of different brand or generic products
dispensed over the life of the prescription were calculated. Using this method, a maximum
of five switches per prescription and six different products supplied over the life of the
prescription was possible.
6.3.3 Methods: Part 3 - Number of brand switches per patient; a “patient-
level” analysis
In Part 3 of the study, a retrospective cohort design was used to examine the number of
switches per patient for the entire duration of an episode of medicine use; a “patient-level”
analysis. Patients who received any of the study medicines during the five year study
period were eligible for inclusion. Follow-up for each study medicine used by a patient
began on the first dispensing post the study start date and continued until discontinuation
92
(defined as more than ninety days since the last dispensing for that medicine), patient death
or study end; whichever was reached first. Patients with only one dispensing of a study
medicine were excluded from the final analysis.
Switches were identified if a patient received different brand or generic products of the
same strength medicine at consecutive dispensings within sixty days (see Table 6.2). If the
manufacturer code was not recorded for a claim, it was assumed that the same product was
supplied at consecutive dispensings. Of the 4,000,948 claims included in Part 3 of the
study, only 3.0% did not have a manufacturer code recorded.
6.3.3(i) Brand substitution status of patients
For each study medicine, patients who received the same product throughout follow-up
were classified as non-switchers. Brand substitution patients were defined as patients who
had only one switch, or had a total of two switches involving a switch and then a switch
back to the original product. Patients with three or more switches, or who had two switches
but received three different products during follow-up were defined as multiple switchers.
To determine the association with switching, the total number of prescribers and
dispensing pharmacies during follow-up was identified for each patient. The product
received by each patient at the initial dispensing during the study period was identified as
being either a co-payment priced product or a product with a brand premium.
6.3.3(ii) Statistical analysis
A multinomial generalised estimating equation was used to compare the proportion of
patients defined as non-switchers, brand substitution patients and multiple switchers for
each medicine. A multivariate multinomial logistic regression model was used to
determine differences in brand substitution status and patient age, gender, duration of
follow-up, number of prescribers and number of dispensing pharmacies. Chi squared tests
were used to compare the brand substitution status of patients who were dispensed co-
payment or premium priced products at their initial dispensing. No adjustments were made
for multiple comparisons. All analyses were undertaken using SAS version 9.1 (SAS Inc.,
Cary, NC, USA).
93
6.4 Results
6.4.1 Results: Part 1 – Background analysis
6.4.1(i) Major PBS listing changes for the study medicines
There were no additions or deletions of multiple bioequivalent products, and no new brand
premium additions during the study period for atenolol, metformin or omeprazole.
In May 2000, eight months prior to the study start date, brand substitution for enalapril first
became possible. Three bioequivalent products were initially available for substitution, and
in May 2001 an additional seven bioequivalent enalapril products were PBS listed.113
Brand substitution for citalopram was not possible until August 2001, when the first
bioequivalent generic product was PBS listed. In November of the same year, a brand
premium was added to the original brand name product. In August 2003, multiple
bioequivalent citalopram products were PBS listed.
Only two ramipril products were available during the study period, and in December 2004
the manufacturer of the product previously listed at the co-payment price sought a higher
price for their product. Simultaneously, the manufacturer of the product previously subject
to the brand premium reduced the price for their brand. The consequences of the pricing
changes were that the benchmark priced product for ramipril changed and the product
initially available for the patient co-payment attracted a brand premium from December
2004 onwards. The product subject to the brand premium prior to December 2004 was
listed at the co-payment price from December 2004 onwards.
6.4.1(ii) Rate of switching
The average rate of switching per one thousand prescriptions dispensed each month
between January 1st 2001 and February 28th 2006 was 23.1 for atenolol (Figure 6.1), 46.1
for enalapril (Figure 6.3), 28.8 for metformin (Figure 6.4) and 36.3 for omeprazole (Figure
6.5).
Between August 1st 2001 and February 28th 2006, when brand substitution for citalopram
was possible, there was an average of 69.8 switches per 1000 citalopram prescriptions
dispensed (Figure 6.2).
Between January 1st 2001 and November 30th 2004, the average rate of switching per 1000
ramipril prescriptions dispensed was 1.4 (Figure 6.6). Following the ramipril brand
premium changes, between December 1st 2004 and February 28th 2006 there was an
average of 50.4 switches per 1000 ramipril prescriptions dispensed.
94
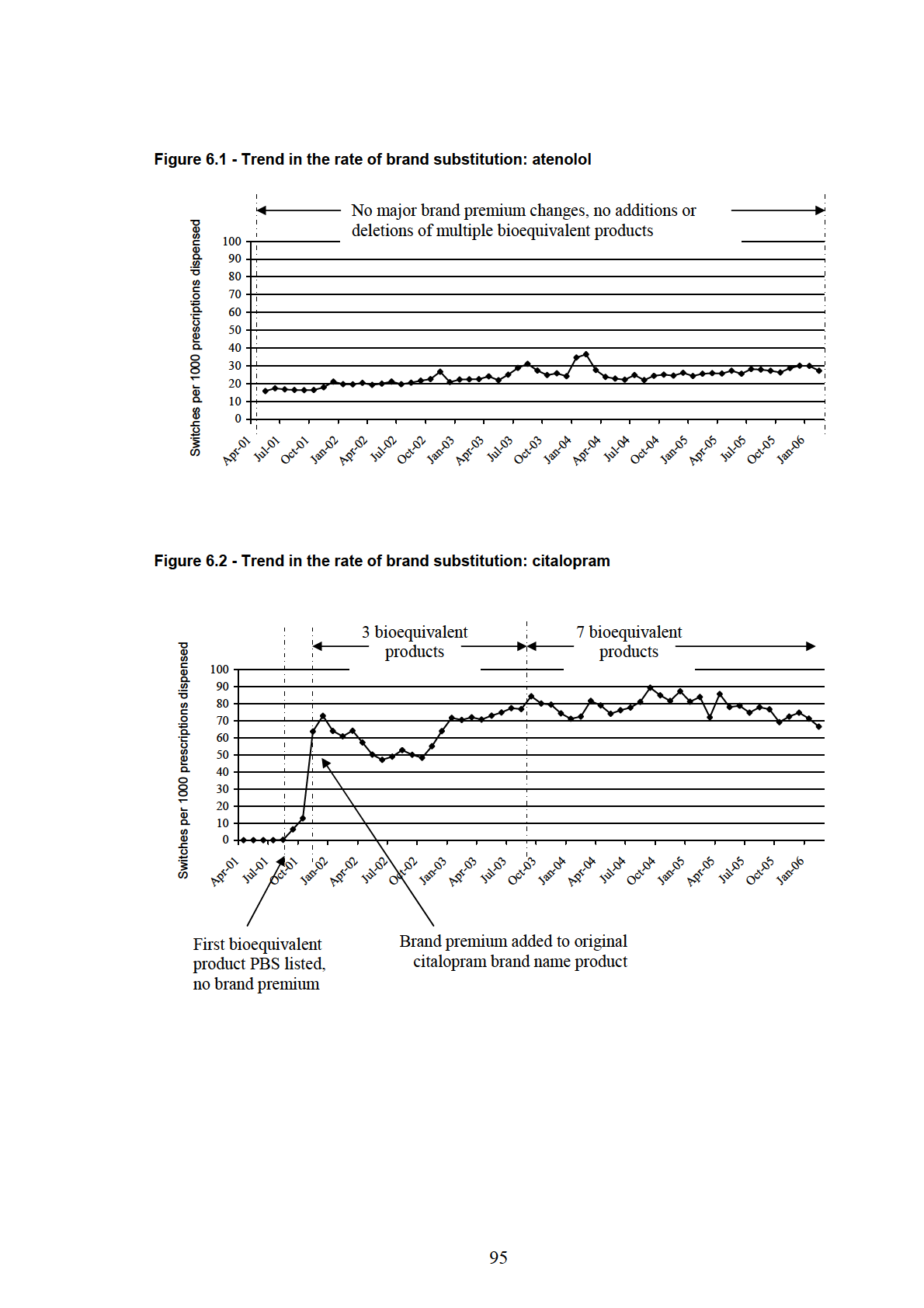
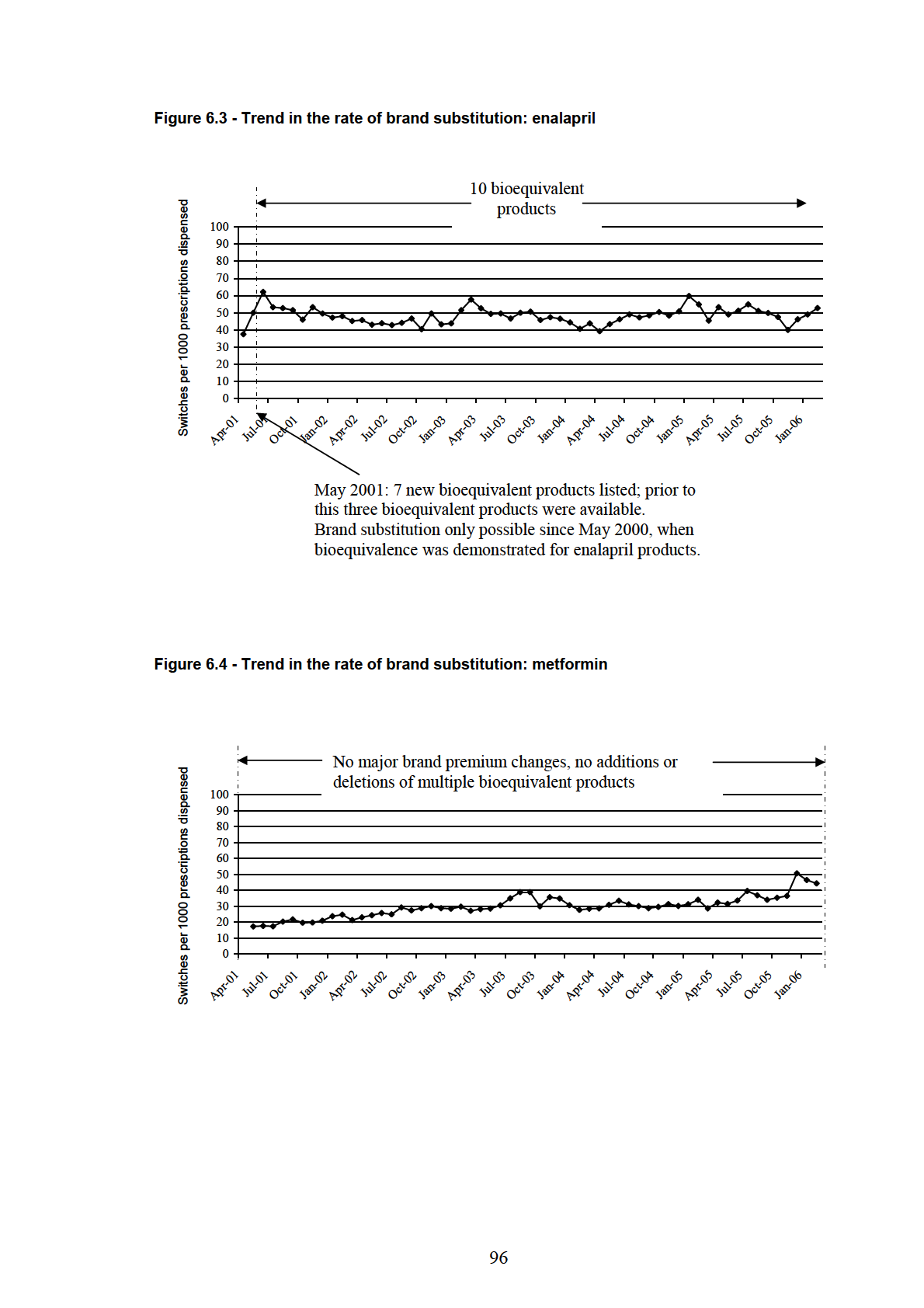
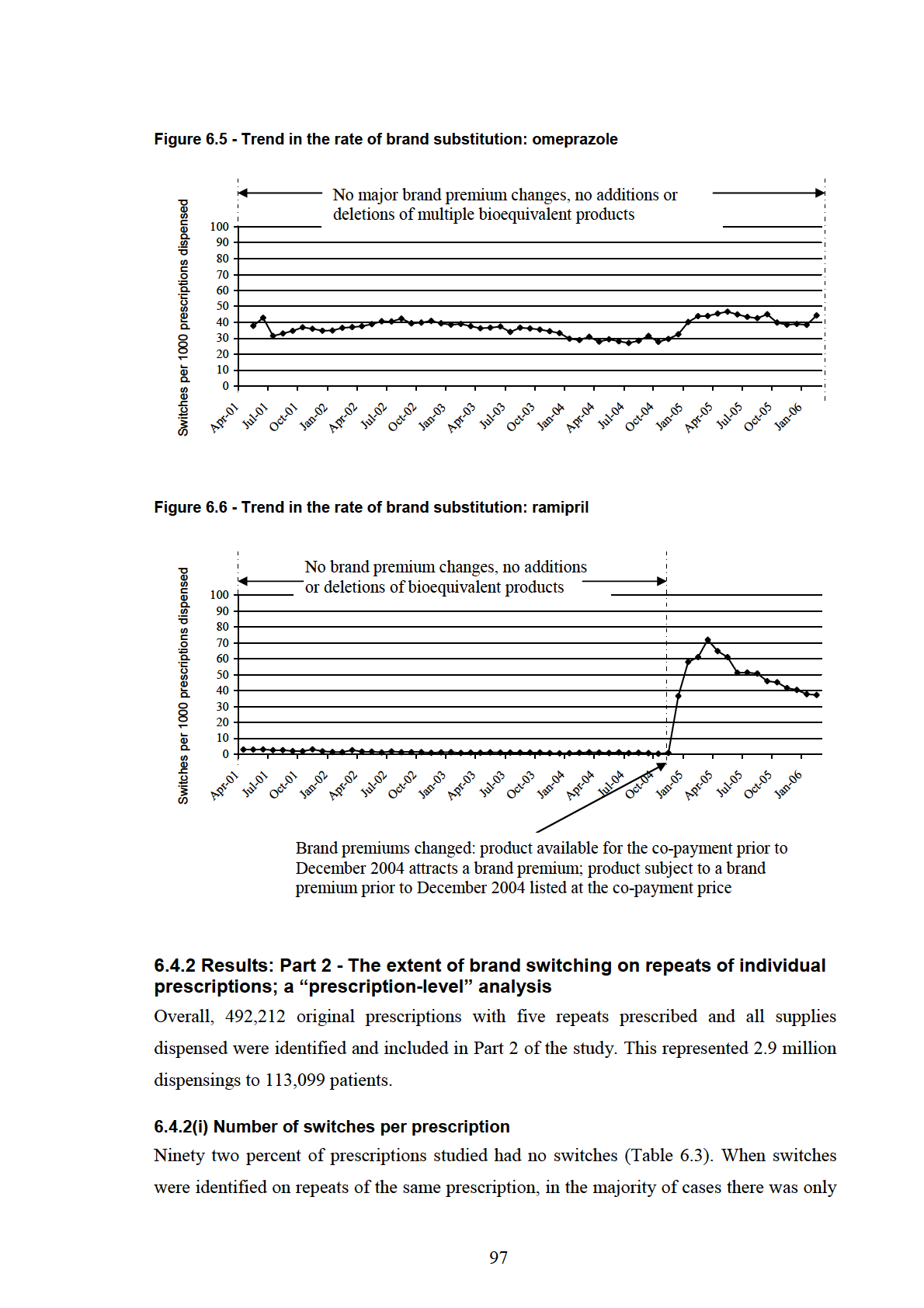
one switch per prescription (6% of all prescriptions). Only 1% of all prescriptions
identified had more than one switch (Table 6.3).
Only 1% (n = 5,961) of the 492,212 prescriptions studied had more than one switch, and
the majority of these (n = 4,917) had 2 switches. Only 0.2% (883) of all prescriptions had
three switches and 0.03% (142) had 4 switches. Of the 492,212 prescriptions studied, only
19 had a switch on every repeat dispensing (i.e. five switches per prescription).
Table 6.3 - Prescriptions with and without switches
Study medicine
Number of prescriptions with:
(number of
More than one
prescriptions)
No switches
One switch
switch
Atenolol
106,076 (94%)
5,023 (4%)
1,245 (1%)
(112,344)
Citalopram
27,581 (83%)
4,610 (14%)
1,144 (3%)
(33,335)
Enalapril
42,244 (89%)
4,053 (9%)
1,081 (2%)
(47,378)
Metformin
49,086 (93%)
3,083 (6%)
735 (1%)
(52,904)
Omeprazole
164,681 (93%)
11,978 (7%)
1,284 (0.7%)
(177,943)
Ramipril
65,140 (95%)
2,696 (4%)
472 (0.7%)
(68,308)
Total
454,808 (92%)
31,443 (6%)
5,961 (1%)
(492,212)
In most cases prescriptions with more than one switch had only two different products
dispensed over the life of the prescription (Table 6.4). None of the prescriptions included
in the study had a different product supplied with each dispensing.
Table 6.4 - Number of products dispensed on prescriptions with multiple switches
Study
Prescriptions Number of different brand or generic products dispensed:
medicine
with > one
switch
2
3
4
5
6
Atenolol
1,245
911
323
11
0
0
Citalopram
1,144
682
436
23
3
0
Enalapril
1,081
677
375
26
3
0
Metformin
735
547
184
3
1
0
Omeprazole*
1,284
1,231
53
-
-
-
Ramipril*
472
472
-
-
-
-
Total
5,961
4,520
1,371
63
7
0
*only two ramipril products and three omeprazole products were available for substitution
98
6.4.3 Results: Part 3 - Number of brand switches per patient; a “patient-
level” analysis
160,145 patients met the inclusion criteria for Part 3 of the study. A quarter of these
patients (39,959) were dispensed more than one study drug during follow-up. For all
medicines, the majority of patients were male and the median age was over 81 years. The
median duration of follow-up was between seven and sixteen months (Table 6.5).
Table 6.5 - Gender, age and duration of follow-up for patients included in Part 3 of the study
Median age (years)
Median months follow up
Male patients
on Feb 28th 2006
(interquartile range)
(interquartile range)
Atenolol
24,506 (55%)
82 (79–85)
11 (4–32)
n = 44,575
Citalopram
11,189 (61%)
82 (70–86)
7 (2–19)
n = 18,414
Enalapril
9,123 (58%)
83 (80–87)
16 (5–40)
n = 15,752
Metformin
15,585 (66%)
81 (76–84)
9 (3–26)
n = 23,456
Omeprazole
41,060 (60%)
83 (79–86)
10 (3–28)
n = 67,992
Ramipril
23,358 (63%)
83 (80–86)
8 (2–21)
n = 36,814
6.4.3(i) Brand substitution status of patients
Over 80% of patients using each medicine either had no switches or demonstrated brand
substitution (i.e. had only one switch, or a total of two switches involving a switch and
then a switch back to the original product) (Table 6.6). Multiple switchers using atenolol,
citalopram, enalapril, metformin and omeprazole had a median of 4 (interquartile range 3 –
5) switches during follow-up. Ramipril multiple switchers had a median of 3 (interquartile
range 3 – 4) switches during follow-up.
99
Table 6.6 - Brand substitution status of patients included in Part 3 of the study
Non-switchers
Brand substitution
Multiple switchers
Atenolol
35,781 (80%)
5,929 (13%)
2,865 (6%)
n = 44,575
Citalopram
10,991 (60%)
4,201 (23%)
3,222 (17%)
n = 18,414
Enalapril
9,306 (59%)
3,543 (22%)
2,903 (18%)
n = 15,752
Metformin
18,673 (80%)
3,219 (14%)
1,564 (7%)
n = 23,456
Omeprazole
47,017 (69%)
14,639 (22%)
6,336 (9%)
n = 67,992
Ramipril
32,448 (88%)
3,461 (9%)
905 (2%)
n = 36,814
For all of the study medicines, patients were more likely to be non-switchers than to have a
single brand substitution or multiple switches (Table 6.7). Patients who switched were
more likely to have a single brand substitution than to be multiple switchers (Table 6.7).
Table 6.7 - Rate ratio (95% confidence interval) of brand substitution status comparisons for
patients using each medicine
Likelihood of patients being in the brand substitution status category:
Non-switchers vs.
Non-switchers vs.
Brand substitution vs.
brand substitution*
multiple switchers*
multiple switchers*
Atenolol
6.0 (5.9-6.2)
12.5 (12 - 13)
2.1 (2.0–2.2)
Citalopram
2.6 (2.5–2.7)
3.4 (3.3–3.5)
1.3(1.2–1.4)
Enalapril
2.6 (2.5–2.7)
3.2 (3.1–3.3)
1.2 (1.2-1.3)
Metformin
5.8 (5.6–6.0)
11.9 (11.3-12.6)
2.1 (1.9–2.2)
Omeprazole
3.2 (3.1–3.3)
7.4 (7.2–7.6)
2.3 (2.2–2.4)
Ramipril
9.4 (9.1–9.7)
35.9 (34–38)
3.8 (3.6–4.1)
* p<0.0001 for all comparisons
100
6.4.3(ii) Comparison of multiple switchers and non-switchers
Multivariate logistic regression showed small but statistically significant age differences
between multiple switchers and non-switchers, with the risk of being a multiple switcher
increasing with increasing age for all medicines except ramipril (Table 6.8). There were no
gender differences between non-switchers and multiple switchers for patients dispensed
atenolol, citalopram, metformin or omeprazole. Male enalapril patients were 21% more
likely than female patients to have multiple switches rather than no switches (odds ratio
1.214 (95% CI 1.098 – 1.341); p = 0.0002). Male ramipril patients were less likely than
female patients to have multiple switches (odds ratio 0.792 (95% CI 0.688 – 0.911); p <
0.0001). When all other variables in the multivariate model were held constant, the odds of
being a multiple switcher compared to a non-switcher increased significantly with a six
month increase in length of follow-up, increasing number of prescribers and increasing
number of dispensing pharmacies for all medicines (Table 6.8).
Table 6.8 - Factors associated with being a multiple switcher compared to a non-switcher
Odds ratio of being a multiple switcher compared to a non-switcher
for a one unit increase in: (95% CI)
Age
6 months of
Number of
Dispensing
(years)
follow-up
prescribers
pharmacies
Atenolol
1.006
1.240
1.351
1.226
(1.001-1.011)
(1.224-1.256)
(1.312-1.392)
(1.204-1.249)
p = 0.0002
p < 0.0001
p < 0.0001
p < 0.0001
Citalopram
1.014
1.607
1.663
1.695
(1.010-1.019)
(1.571-1.643)
(1.579-1.752)
(1.630-1.763)
p < 0.0001
p < 0.0001
p < 0.0001
p < 0.0001
Enalapril
1.012
1.313
1.355
1.322
(1.006-1.019)
(1.292-1.333)
(1.301-1.412)
(1.282-1.364)
p < 0.0001
p < 0.0001
p < 0.0001
p < 0.0001
Metformin
1.006
1.322
1.358
1.263
(1.000-1.013)
(1.297-1.347)
(1.303-1.415)
(1.227-1.300)
p = 0.0433
p < 0.0001
p < 0.0001
p < 0.0001
Omeprazole
1.012
1.349
1.552
1.338
(1.008-1.015)
(1.336-1.363)
(1.518-1.588)
(1.316-1.361)
p < 0.0001
p < 0.0001
p < 0.0001
p < 0.0001
Ramipril
0.992
1.342
1.227
1.136
(0.983-1.000)
(1.313-1.373)
(1.170-1.286)
(1.102-1.171)
p = 0.0001
p < 0.0001
p < 0.0001
p < 0.0001
101
6.4.3(iii) Comparison of multiple switchers and brand substitution patients
For all of the study medicines, there were no significant differences in the age or gender of
patients with a single brand substitution and multiple switchers. The odds of being a
multiple switcher compared to having a brand substitution were significantly greater for
patients with longer follow-up and increasing number of prescribers and dispensing
pharmacies (Table 6.9).
Table 6.9 - Factors associated with being a multiple switcher compared to having a single
brand substitution
Odds ratio of being a multiple switcher compared to having
a brand substitution for a one unit increase in: (95% CI)
6 months of
Number of
Dispensing
follow-up
prescribers
pharmacies
Atenolol
1.130
1.063
1.069
(1.114-1.147)
(1.031-1.097)
(1.049-1.089)
Citalopram
1.293
1.200
1.255
(1.267-1.320)
(1.149-1.254)
(1.216-1.295)
Enalapril
1.138
1.103
1.196
(1.119-1.156)
(1.061-1.146)
(1.162-1.232)
Metformin
1.139
1.125
1.074
(1.115-1.163)
(1.078-1.174)
(1.045-1.103)
Omeprazole
1.155
1.151
1.109
(1.143-1.166)
(1.128-1.174)
(1.093-1.125)
Ramipril
1.052
1.075
1.067
(1.027-1.078)
(1.022-1.130)
(1.034-1.101)
*p<0.0001 for all comparisons
102
6.4.3(iv) Brand substitution status and product received at initial dispensing
Patients who received a product with a brand premium at their initial dispensing were more
likely to have multiple switches during follow-up than patients who received a co-payment
priced product (Table 6.10).
Table 6.10 - Product supplied at initial dispensing and number of switches during follow-up
Study
Initiation product:
Patients defined as:
Chi-
Medicine
Non-
Brand
Multiple
square*
switchers
substitution
switchers
Co-payment priced
28,784
3,991
1,778
(n =34,553)
(83%)
(12%)
(5%)
922.9853
Atenolol
Premium priced
6,997
1,938
1,087
p < 0.0001
(n = 10,022)
(70%)
(19%)
(11%)
Co-payment priced
4,750
1,789
1,064
(n = 7,603)
(62%)
(24%)
(14%)
110.5847
Citalopram
Premium priced
6,241
2,412
2,158
p < 0.0001
(n = 10,811)
(58%)
(22%)
(20%)
Co-payment priced
1,990
625
398
(n = 3,013)
(66%)
(21%)
(13%)
90.7775
Enalapril
Premium priced
7,316
2,918
2,505
p < 0.0001
(n = 12,739)
(57%)
(23%)
(20%)
Co-payment priced
13,876
2,034
922
(n = 16,832)
(82%)
(12%)
(5%)
303.2931
Metformin
Premium priced
4,797
1,185
642
p < 0.0001
(n = 6,624)
(72%)
(18%)
(10%)
Co-payment priced
20,755
3,993
1,282
(n = 26,030)
(80%)
(15%)
(5%)
2307.6910
Omeprazole
Premium priced
26,262
10,646
5,054
p < 0.0001
(n = 41,962)
(63%)
(25%)
(12%)
Co-payment priced
29,838
2,699
791
(n = 33,328)
(90%)
(8%)
(2%)
722.8850
Ramipril
Premium priced
2,610
762
114
p < 0.0001
(n = 3,486)
(75%)
(22%)
(3%)
* df = 2 for each study medicine
103
6.5 Discussion
The results of this study confirmed that although brand substitution occurs for the study
medicines, most prescriptions and most patients do not have multiple switches. Only 1% of
prescriptions and less than 20% of patients using each of the study medicines had multiple
switches over the five year study period.
6.5.1 Rate of switching between brand and generic products
The results of the first part of this study showed that brand substitution occurs for the six
study medicines. The study medicines were from a range of therapeutic classes, had
varying numbers of substitutable products and brand substitution had been possible for
varying lengths of time. McManus and colleagues showed that a significant number of
patients switched to using co-payment priced products in the three months post
introduction of a brand premium and new generics for ranitidine and fluoxetine.75 Only a
single switch in the time period immediately following introduction of the brand premium
was considered in their research. Results of the present study confirm that brand
substitution occurs for a wider range of government subsidised medicines than originally
investigated by McManus and that substitution is sustained over longer periods of time
after brand substitution first becomes possible. Because the majority of patients in the
present study did not have multiple switches, it is likely that the rate of switching for each
of the study medicines is related to switches by incident users and first-time switchers.
Brand substitution had been possible for atenolol, metformin, omeprazole and ramipril for
at least three years prior to the study period. Existing users of these medicines had the
opportunity to substitute products prior to the study start date. In contrast, brand
substitution only became possible for citalopram during the study period and for enalapril,
eight months prior in May 2000. For both of these medicines, there were higher overall
rates of switching, over 40 switches per 1000 prescriptions dispensed over the five years of
follow-up. The rate of switching for ramipril after the brand premium changes was also
more than 40 switches per 1000 prescriptions dispensed. Trends in the rate of switching for
citalopram and for ramipril after the brand premium changes followed similar patterns to
the brand substitution trend for simvastatin shown in Chapter 5. In contrast, the rate of
brand substitution for atenolol, metformin and omeprazole was less than 40 switches per
1000 prescriptions dispensed. These findings suggest that brand switching may be most
likely to occur when generics are listed for a medicine for the first time and when there are
major brand premium changes for a medicine.
104
6.5.2 The extent of brand switching on repeats of individual prescriptions;
the “prescription-level” analysis
The extent of switching on repeats of individual prescriptions was analysed in Part 2 of the
study, because current rules of the brand substitution policy do not limit the number of
switches per prescription. This led the Australian Divisions of General Practice (now
known as the Australian General Practice Network) to express concerns that patients may
receive a different product each time their prescription repeats are dispensed.4 They stated
that:
− “Currently, pharmacists are able to change brands every time a consumer
gets a repeat prescription filled, if the doctor has authorised brand
substitution on the script. For patients who have a script with five repeats
this can mean receiving six different versions of the same drug over the life
of the script. This can increase consumers’ confusion about their
medication, particularly for people taking multiple medications which may
all change brand every time they fill a repeat prescription.”4
For 92% of the prescriptions studied in Part 2, the same product was supplied on each
repeat. If switches occurred, there was only one switch per prescription in the majority of
cases (6% of prescriptions). Similarly, multiple products were rarely supplied over the life
of a prescription, even if there were multiple switches on that prescription. None of the
492,212 prescriptions included in Part 2 of this study had a different product supplied on
each repeat dispensing.
The number of switches per prescription and the total number of different products
supplied per prescription was differentiated because the study medicines had different
numbers of products available to switch between. Only two ramipril products were
available, however a patient could potentially alternate between each product with each
repeat dispensing. Although the medicines with the fewest products available (ramipril and
omeprazole) had the lowest proportion of prescriptions with multiple switches (0.7%),
medicines with multiple products available also had a small proportion of prescriptions
with multiple switches. Only 3% of citalopram prescriptions, 2% of enalapril prescriptions
and 1% of atenolol and metformin prescriptions had more than one switch, and in most
cases only two different products were supplied over the life of the script. These four
medicines all had seven or more products available to switch between for the majority of
the study period. The results of Part 2 of the study suggest that a formal limit to the number
of switches per prescription is not required because in most cases the same product is
already supplied over the life of a prescription.
105
6.5.3 The number of brand switches per patient; the “patient-level” analysis
Part 3 of the study, which examined the number of brand switches per patient for the total
duration of medicine use (until therapy cessation or patient death), showed that at least
80% of patients using the study medicines did not switch products or demonstrated brand
substitution. Patients who switched products were more likely to have a single brand
substitution rather than multiple switches. Although concerns have been raised that
multiple switches occur4-8 results of Part 3 of this study suggest that for the majority of
patients (over 80%) the brand substitution policy is not facilitating multiple switches over
the duration of use of a medicine.
Research conducted with consumers using multiple medicines has suggested that confusion
from multiple brand substitutions may lead to poor compliance, or “double dosing” with
two different brands of the same medicine;9 outcomes which would be inconsistent with
Australia’s policy on the quality use of medicines. The present study is the first to
quantitatively assess the number of switches per patient for patients using the study
medicines. Results of Part 3 of the study show that pharmacists rarely dispense multiple
different products to the same patient, even though the rules of the brand substitution
policy do not prevent this from occurring. Pharmacists appear to consistently supply the
same product to patients in most cases and dispense the same product over the life of most
prescriptions, which is in accordance with the Pharmaceutical Society of Australia
guidelines for brand substitution.153
Although the majority of patients received the same product throughout follow-up,
between 2% and 18% of patients using each study medicine had multiple switches.
Multiple switchers attended more pharmacies and had more prescribers than other patients,
suggesting that continuity of care between healthcare providers as well as consumers may
play a role in multiple switching. Inadequate transfer of information between healthcare
providers, consumers and different healthcare settings can result in poor quality use of
medicines and patient harm.154 To address this problem, the Australian Pharmaceutical
Advisory Council (APAC) has developed guiding principles to achieve continuity in
medication management when patients move between health care settings and providers.154
Although these principles discuss the potential for patient confusion from multiple brand
names and the need to ensure that patients understand changes to brands of their
medicine,154 the issue of multiple brand substitutions is not discussed. Healthcare providers
should assume responsibility for maintaining patients on their regular brand of medicine
wherever possible to minimise the likelihood of confusion from multiple brand switches;
106
particularly when patients are elderly, have vision impairment, and/or use multiple
medicines.9 Patients with multiple health care providers should assume responsibility for
informing their pharmacists and prescribers about the product they usually use, to
minimise the likelihood of unnecessary multiple brand switches. Given the results of this
study, consideration should be given for inclusion of this principle in future updates of the
APAC guiding principles document.
One of the reasons for introduction of the minimum pricing policy was to send a price
signal to patients regarding the cost of different brands of the same medicine. Results of
Part 3 of the study suggest that patients respond to this price signal. For each of the study
medicines, patients who received a product with a brand premium at their initial dispensing
were more likely to switch and were more likely to have multiple switches during follow-
up than patients who received a co-payment priced product. While it may be expected that
patients initiated on a product with a brand premium would have a single brand
substitution to avoid paying the brand premium, they were also more likely to have
multiple switches. This suggests that prescribers should consider which product they
prescribe at the initial dispensing, particularly for patients at risk of confusion from
multiple switches.
Patient response to the price signal associated with brand premiums was also demonstrated
in the brand substitution trend for ramipril. Between January 1st 2001 and November 30th
2004, the same two products were available for ramipril and the brand premium did not
change. During this time period, there was an average of only 1.4 brand switches per 1000
ramipril prescriptions dispensed. On December 1st 2004, pricing of ramipril products
changed. The product previously available for the co-payment attracted a brand premium,
and the product previously subject to a brand premium reverted to the co-payment price.
From December 2004 onwards, there was an average of 50.4 switches per 1000 ramipril
prescriptions dispensed. It is likely that the increase in ramipril brand switching in
December 2004 was associated with patient response to the price signal of the brand
premium changes.
Seventeen percent of citalopram patients and 18% of enalapril patients had multiple
switches during follow-up, compared to less than 10% of patients using the other study
medicines. Patients using citalopram and enalapril had less opportunity to switch from
premium priced products prior to the study start date than patients using the other study
medicines, because in both cases bioequivalent generics had only recently been introduced.
This is a possible explanation for the higher proportion of citalopram and enalapril patients
107
with multiple switches compared to the other study medicines. International studies have
shown that patients switch from brand name to generic products when generic products
first become available for a medicine.33,95,98 The study of simvastatin in Chapter 5 showed
that, in the sixteen months following introduction of simvastatin generics, 47% of patients
switched products. Similar to simvastatin users, patients using citalopram and enalapril had
no or limited opportunity for brand substitution prior to the start of the study period. The
patient pool for switching is likely to have been larger for citalopram and enalapril than for
the other study medicines, which is a potential explanation for the higher rate of switching
and the greater number of people dispensed these medicines who switched.
6.5.4 Strengths and limitations of the study
One of the strengths of this study is the five year study period and the large sample size.
With such a long period of follow-up, nearly half a million prescription forms studied and
over 160,000 patients involved it is less likely that random error influenced the findings
than would have been the case if a smaller sample size was studied.155
A potential limitation of the study is underestimation of the number of switches per
prescription and patient, due to the assumption that the same product was supplied at
consecutive dispensings when the manufacturer code was missing for a claim. It is possible
that in some cases different products were supplied at consecutive dispensings when
manufacturer codes were not recorded. This is unlikely to have influenced the results to a
large degree, because only 3% of claims included in this study had the manufacturer code
missing.
We considered that a patient had a single brand substitution if they had only one switch, or
had two switches involving a switch and then a switch back to the original product. This
second caveat was included because there may be valid reasons for a patient to switch
more than once. For example, a patient may switch to a new product but decide that they
prefer the previous product, and so switch back. Patients may request the pharmacist to
dispense a product different to the product supplied at the previous dispensing. The product
usually used by a patient may be unavailable, e.g. if it is out of stock at the pharmacy or
unavailable from the manufacturer. In this situation it is not unreasonable for a patient to
be substituted to a different product to ensure continuation of therapy, and it is also not
unreasonable that the patient would switch back to their usual product when it became re-
available.
108
Switches were identified if different brand or generic products of the same strength of
medicine were supplied at consecutive dispensings within sixty days in Parts 1 and 3 of
this study. The sixty day time limit was not used in the identification of switches in the
prescription-level analysis for Part 2 of the study. Patients may have multiple prescription
forms for their medicine and if different prescription forms are dispensed consecutively,
then the time between consecutive repeat dispensings within an individual prescription
may exceed sixty days. For this reason the time limit was not used when identifying
switches for the prescription-level analysis in Part 2 of the study.
Data for a five year period were available for this study; however the median length of
patient follow-up was less than 16 months. This level of medication persistence reflects
what occurs in practice and is comparable to that seen in other studies.105,156-158 It is
possible that patients using the medicines in this study switched to other treatments or re-
initiated therapy following cessation.158 However, subsequent episodes of medicine use
were not considered for this study.
DVA pharmacy claims data does not include information on whether or not prescriptions
have been marked “brand substitution not permitted”, so it is unclear how often brand
substitution was not possible and the influence that this had on the results. However, recent
research suggests that brand substitution is possible for most prescriptions. A survey of
Australian doctors conducted in 2006 found that the majority of prescribers marked less
than a quarter of their prescriptions “brand substitution not permitted”,90 and a subsequent
survey of pharmacists supported this finding.92
Because the study was limited to DVA treatment card holders, young people were
underrepresented. Only 9% of veterans are aged 55 years or younger159 compared to 76%
of the overall Australian populaton.142 Australian pharmacists have expressed reluctance to
substitute products for patients who they feel may become confused as a result,82 and
elderly patients are in this high risk group.9 Pharmacists may be more reluctant to
substitute products for elderly patients than younger patients, and if this is the case, the
findings of the studies in this chapter may not be generalisable to younger Australians.
However, younger patients have not been identified as a high risk group for confusion
from brand substitution and veterans were therefore a more relevant population within
which to study brand substitution. There is no evidence to suggest the results of this
research are not generalisable to other elderly Australians. All of the medicines studied are
equally available on the PBS and RPBS, and the co-payments paid by DVA card holders
are the same as PBS concession card holders.2 In addition, there is no evidence to suggest
109
that pharmacists are more or less likely to substitute products for DVA card holders than
other elderly Australians. Although DVA treatment card holders have a higher overall rate
of health resource utilisation than other Australians due to their high rate of service
disability,143 elderly Australians and veterans without service related disability have similar
health resource utilisation.143 For these reasons, results of the studies in this chapter are
likely to be generalisable to other elderly Australians.
6.6 Conclusions
One of the reasons for implementation of the minimum pricing policy and brand
substitution was to send a price signal to patients and encourage the use of generics,75 it
was not intended to facilitate multiple switches. For the medicines included in this study,
the brand substitution policy appears to be having its intended effect for over 80% of
patients – that is, allowing choice and providing patients with the opportunity to avoid
paying brand premiums, without facilitating multiple switches. Despite this, some patients
have multiple switches. Results of this study suggested that initial receipt of a product with
a brand premium and people with multiple dispensing pharmacies and multiple prescribers
were at increased risk of having multiple switches.
Results of the study in this chapter showed that multiple switches were not common,
however, only individual medicines were considered. Forty two percent of veterans use
five or more regular medicines141 and brand substitution is possible for over 400 strengths
and formulations of PBS medicine.160 In the next chapter, the issue of multiple switching is
examined amongst a high risk population. The study reported in Chapter 7 of this thesis
will involve a cohort of patients using multiple medicines and the number of switches for
all of the medicines used by these patients will be identified. Patients at risk of having
multiple switches will be further characterised.
110
CHAPTER 7
The extent of brand substitution for patients using
multiple medicines
7.1 Introduction
The study in Chapter 6 showed that, when single medicines were examined, the majority of
patients did not have brand and generic products of their medicine substituted and those
who did were more likely to have a single brand substitution rather than multiple switches.
In this chapter the research is taken a step further by examining the number of brand
substitutions for all of the medicines used by a cohort of patients using multiple medicines.
To address concerns that patients using multiple medicines may be more vulnerable to
confusion when brand substitution occurs multiple times,9 the factors associated with
patients who have multiple switches will be identified.
7.2 Aims
The overall aim of this study was to identify factors associated with people who have
multiple switches. Specific objectives were to:
•
Determine the frequency with which patients have multiple switches for multiple
medicines.
•
Identify the association between patient age, gender, socioeconomic status, remoteness
of residence, living in the community, total number of prescription medicines used,
number of hospital admissions, having a home medicines review, co-morbidity score,
number of pharmacies, number of prescribers and having multiple switches during
follow-up.
111
7.3 Methods
7.3.1 Study period and study medicines
A retrospective cohort study was conducted using DVA administrative claims data for the
fifteen month period from June 1st 2005 until August 31st 2006. All PBS and RPBS
medicines used in the management of chronic conditions for which brand substitution was
possible on June 1st 2005 were included (Table 7.1). Medicines for which brand
substitution was not possible on June 1st 2005, as well as medicines generally used for the
treatment of acute conditions and medicines usually used intermittently were excluded
(Table 7.2).
Information extracted from the DVA pharmacy claim records for each eligible PBS
medicine included a patient identifier, the date of supply, date of prescription, a prescriber
identifier, a dispensing pharmacy identifier, the quantity of medicine supplied and the
manufacturer code (indicating the brand or generic product dispensed).
7.3.2 Patient inclusion criteria
The analysis was limited to DVA gold card holders who had the opportunity for brand
substitution of two or more medicines. This was defined as patients who received two or
more medicines for which brand substitution was possible over the three month period
between June 1st and August 31st 2005; and who received two or more dispensings for each
of those medicines during that time period. The three month time period was selected
because a previous study which matched RPBS dispensing claims to nursing home
medicine chart review found that a twelve week interval of claims data gave an accurate
estimate of current medicine use.161
Brand substitution was assessed for all medicines dispensed to patients over the fifteen
month study period. Patients were followed from their first dispensing for a medicine
within the study period and follow-up ended upon therapy cessation (defined as more than
ninety days since the last dispensing), patient death or the end of the study period (August
31st 2006); whichever occurred first. Medicines with only one dispensing during the study
period were excluded from the final analysis (see Figure 7.1).
112
Table 7.1 - PBS and RPBS medicines eligible for inclusion
Medicine name and strength
Medicine name and strength
allopurinol 300mg
labetalol 100mg, 200mg
alprazolam 250mcg, 500mcg, 1mg, 2mg
lamotrigine 5mg, 25mg, 50mg, 100mg,
200mg
amiodarone 100mg, 200mg
leflunomide 10mg, 20mg
atenolol 50mg
levodopa:carbidopa 100mg:25mg,
250mg:25mg
azathioprine 25mg, 50mg
lisinopril 5mg, 10mg, 20mg
baclofen 10mg, 25mg
medroxyprogesterone acetate 5mg, 10mg
betaxolol eye drops 0.5%
meloxicam 7.5mg, 15mg
brimonidine 0.2% eye drops
metformin 500mg, 850mg, 1g
brinzolamide eye drops 1%
methotrexate 2.5mg
bromocriptine 2.5mg, 5mg, 10mg
methyldopa 250mg
calcitriol 0.25mcg
metoprolol 50mg, 100mg
captopril 12.5mg, 25mg, 50mg
mianserin 10mg, 20mg
carbamazepine 100mg, 200mg
mirtazapine 30mg
carvedilol 3.125mg, 6.25mg, 12.5mg, 25mg
moclobemide 150mg, 300mg
cimetidine 200mg, 400mg, 800mg
nifedipine 10mg, 20mg, 30mg SR, 60mgSR
citalopram 20mg
nitrazepam 5mg
clomipramine 25mg
nizatidine 150mg,300mg
colchicine 500mcg
omeprazole tab 20mg
cyclosporin 25mg, 50mg, 100mg
oxazepam 15mg, 30mg
cyproterone 50mg, 100mg
paroxetine 20mg
diazepam 2mg, 5mg
ketoprofen 200mg
diclofenac 25mg, 50mg
pindolol 5mg, 15mg
dothiepin 25mg, 75mg
piperazine oestrone sulphate 625µg, 1.25µg
diltiazem 60mg, 180mg SR, 240mg SR,
piroxicam dispersible 10mg, dispersible
360mg SR
20mg, 10mg capsule, 20mg capsule
enalapril 5mg, 10mg, 20mg
prazosin 1mg, 2mg, 5mg
famotidine 20mg, 40mg
quinapril 5mg, 10mg, 20mg
felodipine 2.5mg, 5mg, 10mg
ramipril 1.25mg, 2.5mg, 5mg
flecainide 100mg
ranitidine 150mg, 300mg
fluoxetine dispersible 20mg, 20mg tablet
selegiline 5mg
flutamide 250mg
sertraline 50mg, 100mg
fluvoxamine 50mg, 100mg
simvastatin 5mg, 10mg, 20mg, 40mg, 80mg
gabapentin 100mg, 300mg, 400mg, 800mg
sodium valproate 200mg, 500mg
gemfibrozil 600mg
sotalol 80mg, 160mg
glibenclamide 5mg
spironolactone 25mg, 100mg
gliclazide 80mg
sucralfate 1g
glimepiride 1mg, 2mg, 3mg, 4mg
tamoxifen 10mg, 20mg
glipizide 5mg
temazepam 10mg
Hydrochlorothiazide 50mg: amiloride 5mg
ticlopidine 250mg
indomethacin 25mg
timolol eye drops 0.5%, 0.25%
isosorbide mononitrate 60mg, 120mg
trandolapril 500mcg, 1mg, 2mg
pilocarpine eye drops 0.5%, 1%, 2%, 3%,
verapamil 40mg, 80mg, 120mg, 160mg SR,
4%, 6%
240mg SR
113
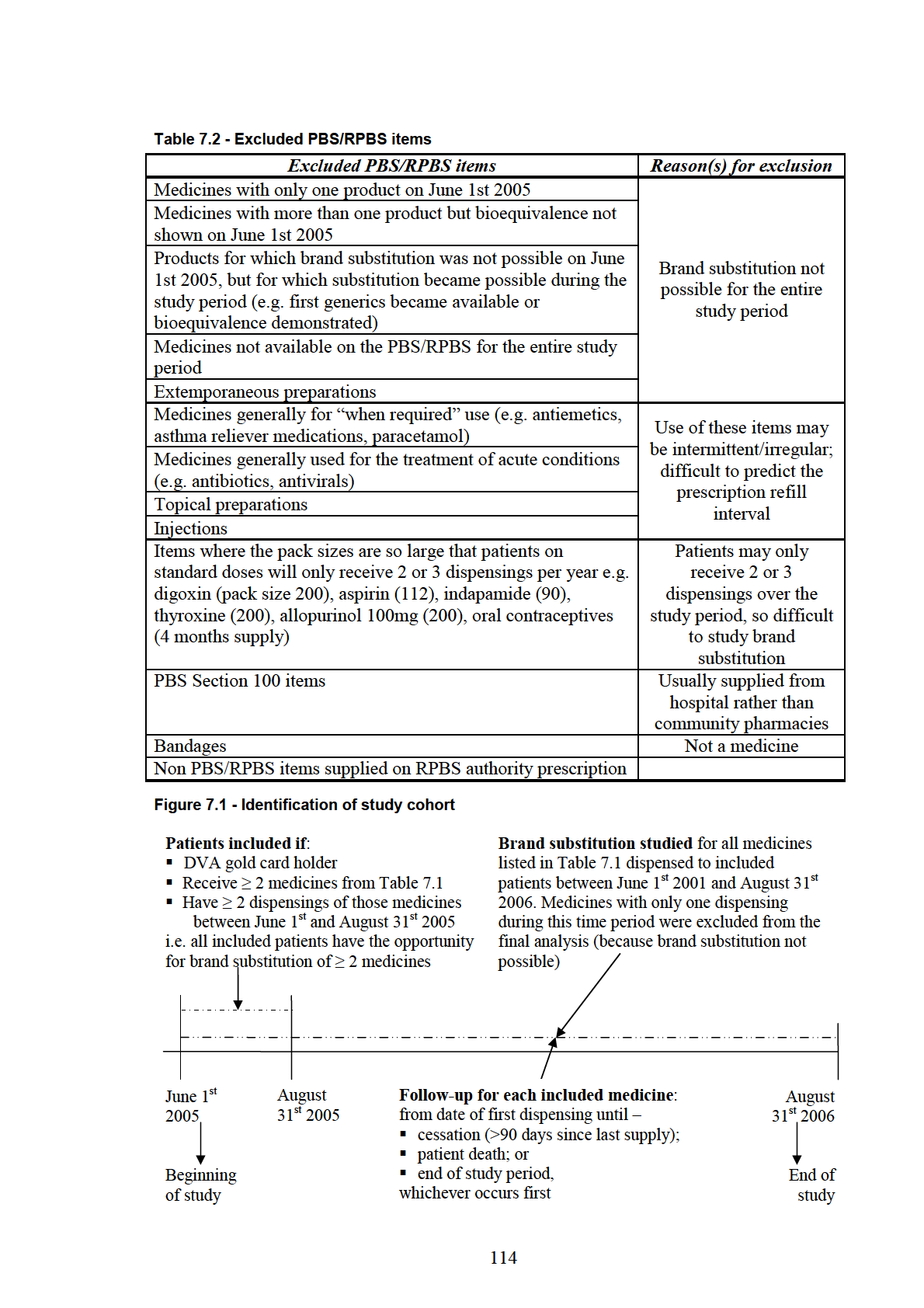
7.3.3 Identification of switches for each included medicine
Switches were identified for each medicine if different brand or generic products were
supplied at consecutive dispensings within 60 days. The 60 day interval was calculated
from the claims data used for this study and 90% of patients returned for repeat medicine
dispensings within this time interval. If the manufacturer code was not recorded for a
claim, it was assumed to be the same code as the previous dispensing. Of the 3.1 million
claims included in this study, the manufacturer code was missing for only 2%.
The number of switches and the number of different brand and generic products dispensed
for each medicine was calculated. If the same product was dispensed throughout follow-up
there were no switches for that medicine. A brand substitution was defined if there was
only one switch for a medicine, or a total of two switches involving a switch and then a
switch back to the original product. A medicine had multiple switches if there were three
or more switches during follow-up, or a total of two switches but three different products
dispensed.
Results were stratified according to the number of switches for all of the medicines
dispensed to a patient. Patients were grouped according to whether they had no switches
for any medicines, a single brand substitution for one or more medicines (but no medicines
with multiple switches), or if they had multiple switches for one or more medicine during
follow-up. Results were further stratified for patients with multiple switches according to
whether they had multiple switches for one medicine, or multiple switches for more than
one medicine.
7.3.4 Identification of patient characteristics associated with multiple
switches
To identify factors associated with patients having multiple switches, information relating
to patient age, gender, length of time in the study, residential aged-care facility status, total
number of prescription medicines used, whether or not the patient had a home medicines
review (HMR) during the study period and the number of hospitalisations during follow-up
was extracted from the DVA administrative claims datasets. Patients who lived in a
residential aged-care facility at any time during the study period were defined as residents,
while patients who were not residents of an aged-care facility during the study period were
defined as living in the community. The total number of prescription medicines dispensed
to a patient (including those for which brand substitution was not possible) was determined
from the DVA pharmacy claims database by the number of unique PBS and RPBS item
codes dispensed over a four month period between June 1st and September 30th 2005. The
115
four month interval was selected to ensure products with large pack sizes (and therefore
longer re-supply intervals) were identified. The total number of private hospital admissions
per patient during follow-up was identified from the DVA private hospital admissions
dataset.
A proxy for socio-economic status was estimated for patients using the index of relative
socio-economic disadvantage (IRSD). IRSD is a validated instrument162 derived from
Australian census data by the Australian Bureau of Statistics (ABS).163 The IRSD is based
on measures including the income of residents in an area, their level of education and the
level of unemployment.163 Low scores on the index reflect relative disadvantage, while
high scores reflect relative lack of disadvantage.163 An area with residents of low income,
low educational attainment and high unemployment would attain a low score on the
IRSD.163 IRSD scores are not recorded for an area if the population in that area is less than
ten people, if few people in the area are employed, or if there is more than a 70% non-
response rate for the census questions used to derive the IRSD scores.163 The most recent
IRSD scores available were based on data from the 2001 Australian census. Patient
postcodes were identified from the DVA client file, and IRSD scores were assigned to
patients based on their postcode of residence on June 1st 2005.
To determine whether location of residence is associated with multiple switches,
remoteness of residence was categorised for the patients in this study based on the
Australian Standard Geographic Classification (ASGC) remoteness area class. The ASGC
remoteness class was developed by the Australian Bureau of Statistics from census data
and is based on the shortest distance by road from an area to the nearest town where goods
and services can be accessed.164 Areas are classified as major cities, inner or outer regional
areas, remote areas or very remote areas. The most recent ASGC remoteness class data
available was from the 2001 census. ASGC remoteness class was assigned to patients
based on their postcode of residence on June 1st 2005.
A co-morbidity score was calculated for each patient using the RxRisk-V.165 The RxRisk-
V uses pharmacy claims data to estimate a patients level of co-morbidity.165,166 Patients are
classed as having a particular therapeutic condition if they receive a medicine mapped to
that condition. A given medicine only appears once in an RxRisk-V class, and does not
contribute to identification of more than one medical condition.165 Higher RxRisk-V scores
indicate increasing co-morbidity.165 RxRisk-V scores for the patients included in this study
were calculated based on the medicines dispensed over the four month period between
June 1st and September 30th 2005.
116
7.3.5 Statistical analysis
Patients were grouped according to the number of switches for each included medicine for
sub-group analyses. Patients who had multiple switches for one or more medicine were
compared with patients who had no switches for any medicines, and patients who had a
single brand substitution for one or more medicine (but no medicines with multiple
switches). Patients with multiple switches for two or more medicines were compared with
patients who had no switches during follow-up. Differences between these groups were
compared using a multivariate multinomial logistic regression model. The following
variables were included in the model: age, gender, length of time in the study,
socioeconomic status, remoteness of residence, residential aged-care facility status, total
number of prescription medicines dispensed, whether or not the patient had a home
medicines review during the study period, the number of hospitalisations during follow-up
and co-morbidity score. No adjustments were made for multiple comparisons. All analyses
were undertaken using SAS version 9.1 (SAS Inc., Cary, NC, USA).
7.4 Results
Just over 84,000 patients met the inclusion criteria for the study (Table 7.3). The average
age of patients was 79 years and just over half were male. The average length of follow-up
was thirteen months. Although patients included in the study were dispensed, on average,
eleven prescription medicines, brand substitution was possible for an average of three
(Table 7.3).
Table 7.3 - Included patients
Patient characteristics
Total included patients
84,040
Mean age (years) ± SD
79.4 ± 8.6
Male gender (%)
45,698 (54%)
Mean unique prescription medicines
11.2 ± 5.7
received per patient ± SD
Mean duration of follow-up (months) 12.8 ± 2.9
± SD *
Mean number of medicines for which 3.4 ± 1.4
brand substitution possible ± SD
*based on duration of follow-up for each included medicine
117
Just under half of the patients included in the study (n = 40,873, 49%) received the same
product throughout follow-up for each medicine. A further 28,657 patients (34%) had a
single brand substitution for one or more included medicines, but had no medicines with
multiple switches during follow-up. Seventeen percent of included patients (n = 14,510)
had multiple switches for at least one included medicine.
Over 80% of the 14,510 patients with multiple switches had multiple switches for only one
medicine. A further 16% had multiple switches for two medicines. Only 475 of the 14,510
patients (3%) had multiple switches for more than two medicines during follow-up (Table
7.4).
Table 7.4 – Patients with multiple switches: number of medicines with multiple switches
Patients with multiple switches
n = 14,510
Number of medicines
Number of
with multiple switches:
patients:
One
11,723 (81%)
Two
2,312 (16%)
Three
416 (3%)
Four
52 (0.4%)
Five
5
Six
2
7.4.1 Multivariate analysis: comparison of patients with multiple switches
and patients with no switches
Multivariate analysis showed that the odds of having multiple switches for one or more
medicine compared to having no switches for any medicines were significantly greater for
patients with increasing number of prescription medicines, hospital admissions, co-
morbidities (measured by RxRisk-V), prescribers, dispensing pharmacies and months of
follow-up; and for patients who lived in major cities compared to inner or outer regional
areas (Table 7.5). The odds of having multiple switches compared to no switches were
lower for older patients, and men were less likely than women to have multiple switches
compared to no switches. Compared to aged-care facility residents, people living in the
community were less likely to have multiple switches (Table 7.5). Having a home
medicines review during follow-up was not significantly associated with having multiple
switches compared to no switches.
118
Table 7.5 - Multivariate comparison of patients with multiple switches for one or more
medicine and patients with no switches for any medicine
Patients with:
Odds ratio(95% CI)
No switches for
Multiple switches
(Reference category:
any included
for at least one
patients with no switches
medicines
included medicine
for any medicine)
n = 40,873
n = 14,510
Mean age ± SD
79.7 ± 8.3
78.8 ± 9.2
0.996 (0.993 - 0.999);
p = 0.0021*
Gender:
Male
22,363 (55%)
7,601 (52%)
0.95 (0.91 – 0.99); p = 0.019
Female
18,510 (45%)
6,909 (48%)
1
Mean total Rx
10.5 ± 5.3
12.4 ± 6.3
1.026 (1.021 – 1.031);
medicines ± SD
p < 0.0001*
Patients who lived in
36,032 (88%)
12,112 (84%)
0.548 (0.515 – 0.583);
the community:
p < 0.0001
Place of residence:†
Major city
25,105 (61%)
9,215 (64%)
1
Inner regional area
11,106 (27%)
3,843 (27%)
1.08 (1.03-1.14); p=0.0027
Outer regional area
4,234 (10%)
1,308 (9%)
1.13 (1.05-1.22); p=0.001
Remote area
314 (0.8%)
103 (0.7%)
1.09 (0.85-1.40); p=0.49
Very remote area
52 (0.1%)
24 (0.2%)
0.83 (0.49-1.39); p=0.47
Socioeconomic status
#
quartile range:#
Lowest 0 – 25%
9,232 (23%)
3,151 (22%)
1.08 (1.01-1.15); p=0.0167
26 – 50%
10,790 (26%)
3,824 (26%)
0.98 (0.92-1.04); p=0.48
51 – 75%
8,904 (22%)
3,158 (22%)
1.03 (0.97-1.10); p=0.295
Top 76 – 100%
11,619 (28%)
4,268 (29%)
1
Patients with HMR
1,882 (4.6%)
891 (6.1%)
0.947 (0.865 – 1.037);
during study period
p = 0.239
Mean
0.78 ± 1.3
1.3 ± 1.7
1.100 (1.082 – 1.114);
hospitalisations
p < 0.0001*
during follow-up
Mean RxRisk-V
4.2 ± 4.1
5.3 ± 4.5
1.024 (1.018 – 1.031);
± SD
p < 0.0001*
Mean pharmacies
1.6 ± 1.1
2.9 ± 1.9
1.664 (1.638 – 1.691);
attended ± SD
p < 0.0001*
Mean prescribers ±
2.0 ± 1.1
2.9 ± 1.9
1.281 (1.261 – 1.301);
SD
p < 0.0001*
Mean months
12.4 ± 3.4
13.5 ± 1.7
1.204 (1.197 – 1.233);
follow-up ± SD
p < 0.0001*
*Odds ratio of having multiple switches compared to no switches for a one unit increase in
this variable
†Odds ratio of having multiple switches for patients living in major cities compared to
other areas
#Odds ratio of having multiple switches for patients in the top 76 – 100% for
socioeconomic status compared to other ranges
119
7.4.2 Multivariate analysis: comparison of patients with multiple switches
and patients with a single brand substitution (but no medicines with multiple
switches)
When all other variables in the multivariate model were held constant, the odds of having
multiple switches for one or more medicine compared to having a single brand substitution
for one or more medicines increased significantly with increasing number of prescription
medicines, hospital admissions, dispensing pharmacies, prescribers and months of follow-
up (Table 7.6). Compared to people living in inner and outer regional areas, people living
in major cities were more likely to have multiple switches than a single brand substitution.
Compared to aged-care facility residents, people living in the community were less likely
to have multiple switches. There were no differences in age, gender, socio-economic status
estimate (using the IRSD), likelihood of having a HMR during follow-up or number of co-
morbidities between people who had multiple switches and people who had a single brand
substitution (Table 7.6).
Table 7.6 - Multivariate comparison of patients with multiple switches for one or more
medicine and patients with a single brand substitution for one or more medicines
(continued on next page)
Patients with:
Odds ratio(95% CI)
A single brand
Multiple switches
(Reference category:
substitution for
for at least one
patients with a single brand
≥
1 medicine**
medicine
substitution for one or more
n = 28,657
n = 14,510
medicine )
Mean age ± SD
79.4 ± 8.6
78.8 ± 9.2
0.999 (0.996 – 1.001);
p = 0.428*
Gender:
Male
15,734 (55%)
7,601 (52%)
1.029 (0.99–1.07); p = 0.194
Female
12,923 (45%)
6,909 (48%)
1
Mean total Rx
11.6 ± 5.8
12.4 ± 6.3
1.010 (1.006 – 1.015);
medicines ± SD
p < 0.0001*
Patients who lived in
23,903 (83%)
12,112 (84%)
0.910 (0.857 – 0.965);
the community:
p = 0.002
Place of residence:†
Major city
17,420 (61%)
9,215 (64%)
1
Inner regional area
8,107 (28%)
3,843 (27%)
1.12 (1.06-1.180; p<0.0001
Outer regional area
2,850 (10%)
1,308 (9%)
1.12 (1.04-1.21); p=0.0027
Remote area
210 (0.7%)
103 (0.7%)
1.12 (0.87-1.44); p = 0.38
Very remote area
48 (0.2)
24 (0.2%)
1.01 (0.61-1.67); p = 0.96
*Odds ratio of having multiple switches compared to a single brand substitution for a one
unit increase in this variable
**but no medicines with multiple switches
†Odds ratio of having multiple switches for patients living in major cities compared to
other areas
120
Table 7.6 continued – Multivariate comparison of patients with multiple switches for one or
more medicine and patients with a single brand substitution for one or more medicines
Patients with:
Odds ratio(95% CI)
A single brand
Multiple switches
(Reference category:
substitution for
for at least one
patients with a single brand
≥
1 medicine**
medicine
substitution for one or more
n = 28,657
n = 14,510
medicine )
Socioeconomic status
quartile range:#
Lowest 0 – 25%
6,413 (22%)
3,151 (22%)
1.047 (0.98-1.11); p = 0.14
26 – 50%
7,653 (27%)
3,824 (26%)
0.984 (0.93-1.05); p = 0.60
51 – 75%
6,215 (22%)
3,158 (22%)
1.014 (0.96-1.08); p = 0.65
Top 76 – 100%
8,163 (29%)
4,268 (29%)
1
Patients with HMR
1,715 (6%)
891 (6.1%)
1.031 (0.944 – 1.126);
during study period:
p = 0.497
Mean
1.0 ± 1.5
1.3 ± 1.7
1.038 (1.024 – 1.052);
hospitalisations
p < 0.0001*
during follow-up
Mean RxRisk-V ±
4.9 ± 4.3
5.3 ± 4.5
1.006 (0.9995 – 1.012);
SD
p = 0.070*
Mean pharmacies
2.1 ± 1.3
2.9 ± 1.9
1.290 (1.273 – 1.308);
attended ± SD
p < 0.0001*
Mean prescribers ±
2.4 ± 1.4
2.9 ± 1.9
1.091 (1.075 – 1.106);
SD
p < 0.0001*
Mean months
13.0 ± 2.6
13.5 ± 1.7
1.118 (1.094 – 1.127);
follow-up ± SD
p < 0.0001*
*Odds ratio of having multiple switches compared to a single brand substitution for a one
unit increase in this variable
**but no medicines with multiple switches
#Odds ratio of having multiple switches for patients in the top 76 – 100% for
socioeconomic status compared to other ranges
7.4.3 Multivariate analysis: comparison of patients with multiple switches for
two or more medicines and patients with no switches during follow-up
The odds of having multiple switches for two or more medicines compared to no switches
for any medicines increased with increasing number of prescription medicines, hospital
admissions, co-morbidities (measured by RxRisk-V), pharmacies, prescribers and months
of follow-up (Table 7.7). People who lived in major cities were more likely than people
living in inner or outer regional areas to have multiple medicines with multiple switches.
The odds of having multiple switches for two or more medicines compared to no switches
decreased with increasing age. People living in the community were less likely to have
multiple switches for multiple medicines than patients living in residential aged-care
facilities (Table 7.7). Socioeconomic status (estimated by IRSD), gender and having a
HMR during follow-up were not significantly associated with having multiple medicines
with multiple switches.
121
Table 7.7 - Multivariate comparison of patients with multiple switches for two or more
medicines and patients with no switches for any medicine
Patients with:
Odds ratio (95% CI)
No switches for
Two or more
(reference category:
any included
medicines with
patients with no switches
medicines
multiple switches for any included medicine)
n = 40,873
n = 2,787
Mean age (years) ± SD
79.7 ± 8.3
78.1 ± 9.8
0.991 (0.987 – 0.996);
p = 0.0005*
Gender:
Male
22,363 (55%)
1,436 (52%)
0.974 (0.90–1.06); p = 0.55
Female
18,510 (45%)
1,351 (49%)
1
Mean total Rx
10.5 ± 5.3
13.6 ± 7.0
1.038 (1.029 – 1.047);
medicines ± SD
p < 0.0001*
Patients who lived in
36,032 (88%)
2,294 (82%)
0.484 (0.432 – 0.544);
the community:
p < 0.0001
Place of residence:†
Major city
25,105 (61%)
1,822 (65%)
1
Inner regional area
11,106 (27%)
724 (26%)
1.14 (1.03-1.26); p=0.009
Outer regional area
4,234 (10%)
214 (8%)
1.36 (1.16-1.59);p=0.0002
Remote area
314 (0.8%)
22 (0.8%)
1.11 (0.68-1.81); p=0.68
Very remote area
52 (0.1%)
5 (0.2%)
0.77 (0.30-2.02); p=0.60
Socioeconomic status
#
quartile range:
Lowest 0 – 25%
9,232 (23%)
614 (22%)
1.04 (0.92-1.17); p = 0.51
26 – 50%
10,790 (26%)
725 (26%)
0.94 (0.83-1.05); p = 0.27
51 – 75%
8,904 (22%)
605 (22%)
1.02 (0.91-1.15); p = 0.69
Top 76 – 100%
11,619 (28%)
822 (30%)
1
Patients with HMR
1,882 (4.6%)
187 (7%)
0.920 (0.778 – 1.088);
during study period:
p = 0.330
Mean hospitalisations
0.78 ± 1.3
1.7 ± 2.0
1.150 (1.123 – 1.178);
during follow-up
p < 0.0001*
Mean RxRisk-V ± SD
4.2 ± 4.1
6.0 ± 4.7
1.034 (1.022 – 1.046);
p < 0.0001*
Mean pharmacies
1.6 ± 1.1
3.6 ± 2.3
1.855 (1.814 – 1.897);
attended ± SD
p < 0.0001*
Mean prescribers ± SD
2.0 ± 1.1
3.6 ± 2.4
1.417 (1.383 – 1.452);
p < 0.0001*
Mean months follow-
12.4 ± 3.4
13.6 ± 1.5
1.239 (1.197 – 1.270);
up ± SD
p < 0.0001*
*Odds ratio of having a multiple switches for two or more medicines compared to no
switches for a one unit increase in this variable
†Odds ratio of having multiple switches for two or more medicines for patients living in
major cities compared to other areas
#Odds ratio of having multiple switches for patients in the top 76 – 100% for
socioeconomic status compared to other ranges
122
7.5 Discussion
The results of this study confirm that most patients do not have multiple brand
substitutions of their medicine, even when all medicine use is examined. For the cohort of
patients included in this study, who were dispensed an average of eleven prescription
medicines; only 17% had multiple switches. These findings support the results of Chapter
6 where it was shown that when individual medicines were considered, fewer than 18% of
patients had multiple switches. Although concerns have been raised that there is the
potential for patients to have multiple switches for multiple medicines,4 results of this
study suggest this does not occur frequently. Only 2,787 of the 84,040 patients included in
the study (3.3%) had multiple switches for more than one medicine.
This study identified the group of patients who are at risk of having multiple switches of
their medicine. For each sub-group comparison, the odds of having multiple switches
increased with increasing number of prescription medicines, hospital admissions,
dispensing pharmacies, prescribers and months of follow-up; and living in a major city or a
residential aged-care facility was also consistently associated with increased risk of having
multiple switches. This is the first study which has identified the population at-risk for
multiple switches. Now that these risk factors have been identified they should be targeted
in quality use of medicines interventions to minimise the likelihood of multiple switches
occurring, particularly for the elderly, people with poor eyesight and people using multiple
medicines who may be at higher risk of confusion as a result.9 Gender, age and
socioeconomic status estimate were inconsistently associated with switching. Although for
some comparisons these factors were statistically significantly associated with increased
odds of having multiple switches the differences were small, suggesting that overall, these
factors are unlikely to have a practical influence on switching.
Previous research has shown that people with multiple co-morbidities and people using
multiple medicines are at increased risk of adverse drug events resulting in hospital
admission167,168 and that this group of people is also at increased risk of confusion from
multiple brand substitutions.9 Although the majority of patients in this study did not have
multiple switches, the small proportion that did tended to have the characteristics which
previous research suggests are associated with high risk of adverse drug events and
increased risk of confusion from multiple brand substitutions. The potential for
compromised quality use of medicines as a result of multiple switches may be high in these
at risk patients.
123
The results of this study suggest that factors related to continuity of care are consistent
predictors of whether or not a patient has multiple brand substitutions of their medicine.
The risk of having multiple switches increased with increasing number of prescribers,
dispensing pharmacies and hospital admissions. This finding confirms and extends the
results of the study reported in Chapter 6, which also showed that the odds of having
multiple switches compared to no switches increased significantly with increasing numbers
of prescribers and dispensing pharmacies. Inadequate transfer of information between
healthcare providers, consumers and different healthcare settings can result in poor quality
use of medicines and patient harm.154 Although guiding principles have been developed to
achieve continuity in medication management when patients move between health care
settings and providers,154 the potential for multiple brand substitutions to occur is not
discussed. The potential for brand substitutions to occur during hospital admission has long
been recognised because most Australian public hospitals have a drug formulary which
specifies the medicines that are routinely stocked and supplied to patients.79 Usually, only
one brand of each medicine listed on the formulary is kept in stock, so brand substitution
occurs for many patients during inpatient medication administration and at the time of
discharge if the hospital does not stock the brand of medicine that the patient used prior to
admission. The consistent finding that multiple brand substitutions occur when patients
have multiple dispensing pharmacies, prescribers and hospital admissions suggests that
more emphasis needs to be placed on ensuring that the product usually used by a patient is
identified when prescriptions are written or dispensed, so that multiple brand substitutions
do not unnecessarily occur.
The timing of hospital discharge and multiple switching was not considered in this study,
so it is unclear whether multiple switches occurred before or after hospital admission.
Further research is required in this area. The DVA dataset contains information for private
hospital admissions only; public hospital admissions are not included. It is therefore likely
that the number of hospital admissions per patient for DVA card holders was
underestimated in this study and it is possible that the odds of having multiple switches
with increasing hospital admissions was underestimated.
People living in the community were less likely to have multiple switches than residential
aged-care facility residents; however multiple brand switches for residential aged-care
facility residents are unlikely to compromise quality use of medicines in most cases.
Residents of aged-care facilities tend not to administer their own medicines and most
residential aged-care facilities use dose administration aids to assist nurses in the
124
administration of medicines to residents.169 The potential for patient confusion and adverse
outcomes from multiple brand substitutions to residential aged-care facility residents is
likely to be lower than it is for patients who self administer and manage their own
medications. This may also be an explanation for the increased odds of having multiple
switches for aged-care facility residents. Australian pharmacists have expressed reluctance
to substitute products for patients who they feel may become confused as a result.82 If
pharmacists are aware that a patient is an aged-care facility resident, and therefore unlikely
to administer their own medication, they may be less concerned about the potential for
patient confusion from multiple brand substitutions.
The association between socioeconomic status (estimated by IRSD) and multiple switches
was inconsistent in this study. For example, compared to patients with no switches,
wealthier patients in the top 25% for socioeconomic status were more likely to have one or
more medicines with multiple switches than were patients in the lowest 25% for
socioeconomic status. However, when patients who had multiple medicines with multiple
switches were compared to patients with no switches during follow-up, there was no
socioeconomic status association. This suggests that socioeconomic status may not have a
meaningful influence on the likelihood of brand switching for Australian patients. Studies
conducted in North America have shown that poorer patients are more likely to receive
prescriptions allowing generic substitution than more wealthy patients.170-172 It is possible
that socioeconomic status has less of a consistent impact on brand substitution for patients
in Australia than it does internationally because of the relative lower cost of medicines in
Australia. The Australian patients in the present study paid $4.70 per PBS prescription,173
and the extra charge for premium priced products was between $1 and $4 for most
medicines.3 In contrast, in 2006 the average price difference between brand name and
generic medicines in the United States was $65.174 The larger price difference between
brand and generic medicines in America may mean that there is a greater financial
incentive for American patients to substitute to generic products. In addition, American
patients of lower socioeconomic status who receive medicines subsidised through
Medicaid may be required to receive generic products if a generic is available.175 Each
state of America runs a Medicaid program, and in 2003 forty one states had “mandatory
generic substitution” policies meaning that pharmacists had to dispense generics to
Medicaid recipients unless the prescriber prohibited substitution for that prescription.175
Using the IRSD as a proxy for socioeconomic status in the present study may also have
125
contributed to the lack of a clear association. Results may have differed if patient-level
socioeconomic status data were used.
Concerns that patients using multiple medicines have multiple switches for many of their
medicines4,7,84,87,149 appear to be unwarranted. Brand substitution is possible for over 400
strengths and formulations of government subsidised medicine,160 and 184 of these were
included in the present study. Given that brand substitution is possible for such a large
number of medicines, and given that study participants were dispensed an average of
eleven government subsidised medicines, it was an unexpected finding that patients were
dispensed on average only three medicines for which brand substitution was possible. This
suggests that many of the medicines dispensed to patients were for the treatment of acute
conditions or medicines usually used on a “when required” basis, and that many medicines
dispensed to patients were still on patent. In June 2005, at the start of the study period, over
800 strengths and formulations of PBS medicine had no bioequivalent substitutes,151 so it
is likely that many patients in the study were dispensed medicines without the opportunity
for brand substitution. Although there are anecdotal concerns that the potential for patient
confusion may be worse when there are multiple switches for multiple medicines,4,7,84,87,149
results of this study suggest multiple switches for multiple medicines occur infrequently.
Only 3% of the 84,040 patients included in this study had multiple switches for more than
one medicine.
One of the strengths of this study is the large sample size. Over 84,000 patients were
included, meaning that the potential for random error to influence the findings is less than
would have been the case if a smaller sample size was studied.155 Similar to the studies
reported in Chapters 5 and 6, a potential limitation of the present study is underestimation
of the number of switches per patient, due to the assumption that the same product was
supplied at consecutive dispensings when the manufacturer code was missing for a claim.
However, this is unlikely to have influenced the results to a large degree because only 2%
of included claims had the manufacturer code missing. The association between multiple
switches and polypharmacy may also have been underestimated because only prescription
medicines were included in the estimate of the total number of medicines used by patients
in this study. Over-the-counter and complementary medicines are used by many veterans141
and could not be included in the count for total number of medicines because no data for
supply of these medicines appears in the DVA datasets. A fifteen month study period was
selected, which is consistent with the median length of patient follow-up for the medicines
126
studied in Chapter 6. This level of medication persistence has also been reflected in other
studies and for other medicines.105,156-158
Similar to the studies reported in Chapter 6, young people were underrepresented in the
study reported in this chapter and the results may therefore not be generalisable to younger
people. However, there is no evidence to suggest the results of this study are not
generalisable to other elderly Australians. All of the medicines studied are equally
available on the PBS and RPBS, and the co-payments paid by DVA card holders are the
same as PBS concession card holders.2 In addition, there is no evidence to suggest that
pharmacists are more or less likely to substitute products for DVA card holders than other
elderly Australians.
7.6 Conclusions
This study showed that most patients do not have multiple brand substitutions of their
medicine, even when all of the medicines dispensed to patients are considered. Factors
independently associated with multiple switches were increasing numbers of prescription
medicines, hospital admissions, prescribers and dispensing pharmacies and length of
follow-up, and living in residential aged-care facilities and major cities. Identification of
the population at risk of having multiple switches will allow QUM interventions to be
appropriately targeted to these high risk groups and would be likely to minimise the
potential for patient confusion as a result.
127
CHAPTER 8
Discussion and conclusions
The studies in thesis are the first to consider the extent of brand substitution for a wide
range of medicines and people and show that implementation of brand substitution in
Australia occurs in a manner consistent with the principles of Australia’s National
Medicines Policy. Over 80% of patients included in this research did not switch products
or had only a single brand substitution for their medicine. Patients who received a product
with a brand premium at their initial dispensing were more likely to have products
substituted than others, suggesting that the brand substitution policy is an important
mechanism to facilitate access to cheaper medicines by consumers. At the same time,
amongst patients who had products substituted, it was more common to have a single
brand substitution than it was to have multiple switches, which was what was intended
when the brand substitution policy was planned. When individual prescription forms were
considered, the majority had the same product dispensed over the life of the prescription
form and if switches occurred there was nearly always only one switch per prescription.
The results of the studies in this thesis suggest that, on the whole, the minimum pricing
policy and brand substitution are implemented in a manner supportive of Australia’s
National Medicines Policy and QUM. They facilitate consumer access to cheaper
medicines without resulting in multiple switches for most patients.
Another positive finding from this research was that, in the case of simvastatin, the overall
rate of brand substitution was no different when ten substitutable products were available
compared to when four substitutable products were available. Although this was only one
case example, it suggests that availability of multiple products does not result in increased
brand switching. Further evidence to support this hypothesis was provided in Chapter 6,
where ten substitutable products were available for atenolol and metformin and fewer than
7% of patients using these medicines had multiple switches. The number of PBS medicines
with substitutable products has increased in recent years. In August 2000, 269 strengths
128
and formulations of PBS medicine had multiple bioequivalent products and there was an
average of three substitutable products per medicine (range 2 – 11).176 In comparison,
seven years later in November 2007, 404 strengths and formulations of PBS medicine had
substitutable products and there was an average of four bioequivalent products per
medicine (range 2 – 15).160 As time passes and more medicines come off patent, the
number of medicines with substitutable products may increase and it is likely that these
medicines will continue to have multiple bioequivalent products for substitution. Results of
this research suggest that even when multiple bioequivalent products are available for a
medicine; this does not result in multiple switches for the majority of patients.
The results of this thesis provide support for continued implementation of brand
substitution. This is important because it is likely that there will be greater financial
incentive for patients to switch from premium to co-payment priced products in the future.
Patient co-payments increase at the beginning of each calendar year and have been shown
to present a barrier to access to medicines by some Australians.68-70 Brand premiums, on
top of yearly co-payment increases, may present an even greater financial barrier to access
to medicines. The minimum pricing policy and brand substitution will continue to be
important mechanisms to ensure that both the government and patients do not pay more
than necessary for medicines and to reduce the financial burden for patients associated
with brand premiums.
8.1 Patients at risk of having multiple brand substitutions
The results of the studies in this thesis show that, at the policy level, implementation of
brand substitution is consistent with the goals of Australia’s National Medicines Policy.
However, 17% of the veterans included in the study in Chapter 7 had multiple switches.
There are 2.7 million Australians aged 65 years or over,142 so if 17% of the elderly
Australian population had multiple switches this would equate to around 450,000 elderly
people with multiple switches of their medicine. Results of this research showed that the
patients most at risk of having multiple brand substitutions tended to have:
•
longer duration of follow-up;
•
products with a brand premium dispensed at their initial supply;
•
more prescribers;
•
more dispensing pharmacies;
•
more prescription medicines;
•
more hospital admissions;
129
•
higher co-morbidity scores;
and were more likely to live in:
•
a residential aged-care facility (rather than in the community)
•
a major city (rather than an inner or outer regional area).
Trends in the rate of brand substitution for individual medicines also suggested that brand
switching was more likely to occur when generics were listed for a medicine for the first
time (e.g. in the cases of simvastatin and citalopram) and when there were major brand
premium changes (e.g. in the case of ramipril).
8.1.1 Strategies to avoid multiple brand substitutions
The studies in this thesis are the first to identify characteristics of people at risk of having
multiple switches of their medicine. Now that the at-risk population has been identified,
quality use of medicines interventions could be targeted towards minimising the likelihood
of the at-risk group having multiple switches. A first step towards this is to inform key
stakeholders in the use of medicines of the characteristics of people at risk of having
multiple switches and to provide them with strategies to avoid multiple switches. The
National Strategy for Quality Use of Medicines identifies key partners whose involvement
is required in order to achieve quality use of medicines. They are: “health care consumers,
their carers and the general community; health practitioners and health educators; health
and aged-care facilities; medicines industries; media; health care funders and purchasers;
and Governments – including Commonwealth, States, Territories, and Local
Governments.”19
The Australian Pharmaceutical Advisory Council (APAC) and the National Prescribing
Service (NPS) could play roles in informing stakeholders of the people at risk of having
multiple switches and informing them of strategies to avoid multiple switches. For
example, results of the studies in this thesis suggest that greater recognition is required of
the potential for multiple brand substitutions to occur when patients move between
different prescribers, dispensing pharmacies, and between health care settings such as
hospital and the community. This information could be incorporated into APAC guiding
principles documents. In 1998, the “National guidelines to achieve the continuum of
quality use of medicines between hospital and the community” were released by APAC177
and were revised and re-released as a guiding principles document in July 2005.154 The
potential for patient confusion from multiple brand names for the same medicine is
highlighted in the APAC guiding principles, as is the need for health professionals to
130
ensure that patients understand any brand changes that occur during an episode of care.154
The need to ensure that patients understand and recognise the difference between the brand
and generic names of their medicine is noted.154 Issues relating to brand substitution, and
the potential for multiple switches across the continuum of care, are not discussed in the
APAC continuity of care guiding principles document and could be incorporated.
In 2003, an APAC working party was established to identify quality use of medicines
issues relating to brand substitution and to develop a quality use of medicines statement on
brand substitution.178 By November 2004, the draft policy statement for safe and effective
brand substitution had been developed179 and was distributed to stakeholders for comment
in November 2005.180 However, in May 2006 APAC noted that the “work was taking a
different approach” and was “currently being redrafted and updated as a guiding principles
document”.181 In September 2007 the document was still in draft form and was not
publicly available (personal communication, Lisa Talevich, APAC secretariat, 18th
September 2007). Findings of this thesis, in particular the characteristics of patients at risk
of having multiple brand substitutions, could be incorporated into the APAC guiding
principles for safe and effective brand substitution. Public release of these guiding
principles should occur without further delay as a mechanism to inform stakeholders of the
at-risk groups for multiple switches.
NPS is another appropriate organisation to inform health care practitioners and consumers
of the at-risk groups for multiple brand substitutions. NPS produces publications for health
professionals,182 which could be used as vehicles within which to inform health care
professionals of the groups at risk of having multiple switches. NPS also provides case
studies, clinical audits and practice audits for GPs and pharmacists which are designed to
assist these health professionals improve their clinical decision making skills.182 Clinical
audits could be used to help health care professionals identify their patients who are at risk
of multiple brand substitutions and to provide strategies for health professionals to use to
minimise the likelihood of multiple switches occurring, particularly when patients are at
high risk of confusion as a result.
NPS consumer information services are a means through which consumers could be
informed of the situations in which multiple switches are likely to occur and strategies to
avoid unnecessary multiple switches. In August 2007, a media campaign was launched by
NPS entitled “Generics are an equal choice” with the aim of increasing awareness,
understanding and acceptance of generic medicines by consumers.182 This campaign was
specifically targeted towards elderly people using multiple medicines.182 Alongside of
131
television advertisements informing consumers about generic medicines, peer educators
were trained to provide information about generic medicines and quality use of medicines
to elderly people as part of the Seniors QUM program run by NPS.182 NPS peer educators
could provide information to elderly consumers on the situations in which multiple brand
substitutions are likely to occur, and could inform consumers on strategies to avoid
multiple brand switches.
In addition to NPS activities and APAC guiding principles documents, information relating
to the at-risk population for multiple switches should be incorporated in professional
practice guidelines such as the Pharmaceutical Society of Australia (PSA) brand
substitution guidelines for pharmacists153 and professional practice standards for
dispensing of medicines,183 the Society of Hospital Pharmacists of Australia (SHPA)
practice standards,184,185 and prescribing guidelines.
In addition to increased awareness of the characteristics of people at risk of having
multiple switches of their medicine, stakeholders should implement strategies to minimise
the potential for multiple switches to occur. For example, results of the studies in this
thesis suggest that if a patient receives a co-payment priced product at their initial
dispensing, they are less likely to have multiple switches than if they received a product
with a brand premium. Prescribers and pharmacists could therefore consider prescribing or
dispensing co-payment priced products at the initial supply for patients at risk of confusion
from multiple brand substitutions. Prescribers have the option of preventing brand
substitution for a prescription. If they are concerned about the potential for patient
confusion from multiple brand substitutions, writing a prescription for a co-payment priced
product and preventing substitution of that prescription would enable at-risk patients to
avoid paying brand premiums, while at the same time reducing the likelihood of multiple
brand substitutions occurring. Home medicines reviews could be conducted for people
with risk factors for having multiple switches, as a mechanism to identify when multiple
switches have occurred and to minimise the potential for patient confusion and adverse
outcomes as a result.
To minimise the likelihood of multiple switches occurring, pharmacists, prescribers and
other health care providers should assume responsibility for identifying the product usually
used by a patient, without assuming that the product last prescribed or dispensed by them is
the only product that has been prescribed or dispensed for the patient. In most cases, this
would be a simple exercise and should involve little more than a conversation with the
patient or their carer, or a phone call to the previous provider. Consumers should assume
132
responsibility for informing their pharmacist or doctor when they visit different pharmacies
and prescribers, to alert them to the potential for multiple switches to occur. To reduce the
likelihood of multiple brand switches occurring, patients at risk of confusion from multiple
switches could consult the same prescriber and dispensing pharmacy wherever possible.
This was a strategy already used by patients who had indicated their concern regarding the
potential for confusion from multiple brand substitutions.81
The pharmaceutical industry is another key stakeholder in the use of medicines19 and could
play a role in reducing the potential for patient confusion when multiple brand
substitutions occur. Common concerns about the potential for confusion from multiple
brand substitutions arise because of the different name and appearance for different brand
and generic products of the same medicine.9,82,89 It has been suggested that medicine
labelling requirements in Australia are inadequate when brand substitution occurs and that
it should be a requirement for the generic medicine name to be more prominent than the
brand name on the dispensed product.89 The pharmaceutical industry could take
responsibility for making generic medicine names more prominent than brand names on
medicine packaging, and the Therapeutic Goods Administration could support this. This, in
conjunction with increased education and awareness amongst consumers regarding the
generic and brand names of their medicine, is a potential strategy to reduce the potential
for patient confusion when brand substitution occurs.
8.2 Continued and future evaluations of the minimum pricing and
brand substitution policies
Continued evaluation of the rate and extent of brand substitution is required to ensure that
the balance between the access and quality use of medicines arms of the National
Medicines Policy is maintained in relation to the brand substitution policy. In May 2007,
the Australian government introduced legislation for PBS reforms, designed to influence
the prices paid by the government for PBS and RPBS medicines.186 Amongst the proposals
were compulsory price decreases for generic medicines, with up to a 25% price reduction
for some medicines with substitutable products.186 To compensate for reduced revenue
associated with the price decreases, from August 2008 the Australian government will pay
a $1.50 incentive to pharmacists each time a co-payment priced product is dispensed for a
medicine with multiple bioequivalent products.187 The government has noted the potential
for this financial incentive to lead to increased use of generic medicines,21 which is likely
to occur via pharmacists offering brand substitution to more patients. Continued evaluation
133
of the rate and extent of brand substitution per patient is necessary to ensure that, following
policy changes like the PBS reforms, implementation of the brand substitution policy
continues to be consistent with the principles of quality use of medicines. Results of this
thesis, in particular the factors associated with people who have multiple brand switches of
their medicine, could be disseminated to pharmacists when this initiative is implemented as
a mechanism to increase awareness of patients most at risk of having multiple switches.
A major basis for the studies in this thesis was concerns regarding the potential for patient
confusion when brand substitution occurs multiple times.4,9 There is little doubt that there
is the potential for multiple brand substitutions to confuse some patients; however the
frequency with which this occurs has not been studied. Qualitative research methods such
as consumer surveys or interviews would be required to determine the prevalence of
confusion resulting from multiple brand substitutions. However, researchers who
conducted interviews with consumers in 2003 regarding their views towards generic
medicines attempted to recruit patients who had experienced confusion from brand
substitution and noted difficulty in doing so.81
Another concern with multiple brand substitutions is the potential for patients to double
dose on more than one brand or generic product if they do not realise that a substituted
product is actually a different medicine.9 It has also been suggested that patients may make
the decision to cease use of a substituted brand or generic product if it looks different to the
tablets they are used to taking.9 Adverse outcomes for patients as a result of multiple brand
substitutions would clearly be at odds with the QUM arm of Australia’s National
Medicines Policy. It was not possible to determine if a patient had double dosed on
multiple brand or generic products of the same medicine from the DVA pharmacy claims
data because dosage information is not recorded. Most medicine dispensings on the PBS
and RPBS equate to one months supply at standard doses. Therefore, if a patient received
two medicine dispensings over a one month period this could indicate double dosing.
However, two medicine dispensings over a one month period may also represent the
dispensing pattern for a patient who takes two tablets daily to make up their daily dose.
Absence of dosage information in the DVA claims data made it impossible to distinguish
between these types of situations. For the same reason, it was also not possible to associate
multiple brand switches with therapy cessation using DVA pharmacy claims data. Because
most PBS and RPBS dispensings equate to one months supply at standard doses, most
patients receive one dispensing each month. However, patients who require only half a
tablet for their daily dose are likely to have re-supply intervals of more than one month.
134
For these reasons, the frequency with which double dosing and therapy cessation are
associated with multiple brand substitutions could not be studied in this thesis. Given the
interplay between the access objective of the National Medicines Policy and the quality use
of medicines, identifying the extent to which double dosing and therapy cessation are
associated with brand substitution is an important area for future research.
8.3 Overall conclusions
Results of the studies in this thesis showed that in most situations and for most patients,
and therefore at the policy level, the brand substitution policy is implemented primarily as
intended. When elderly patients dispensed multiple medicines were considered, over 80%
did not switch products or had only a single brand substitution. When switches occurred, it
was more common to have a single brand substitution per patient rather than multiple
switches. Although concerns have been expressed that there is the potential for multiple
switches to occur under current brand substitution legislation, results of the present
research show that this does not occur frequently.
This research identified characteristics of patients at greatest risk of having multiple
switches. These people tended to have longer duration of follow-up; products with a brand
premium dispensed at their initial supply; more prescribers, dispensing pharmacies,
prescription medicines and hospital admissions; higher co-morbidity scores; and were
more likely to live in a residential aged-care facility or a major city than patients who did
not have multiple switches. Now that this at-risk population has been identified, quality use
of medicines interventions should be targeted in these areas and to these factors, to
increase awareness of when multiple switches occur and to minimise the potential for
unnecessary multiple switches to occur.
The brand substitution policy has operated on the PBS and RPBS for over ten years.
Generic medicines are playing an increasingly important role in the PBS and RPBS and
will continue to do so with the advent of the proposed PBS reforms,186 which provide
financial incentives for pharmacists to encourage increased use of generic medicines.187
Although the potential for patient confusion from brand substitution and multiple brand
substitutions will probably always exist for some patients, the studies reported in this thesis
have provided insight into the extent to which multiple switches occur and the
characteristics of people at risk of having multiple switches. Overall, from the quality use
of medicines perspective, the brand substitution policy is being implemented primarily as
intended for most patients; with the majority of patients either having no switches or only a
135
single brand substitution. Brand substitution is currently implemented in a manner
supportive of the overall objective of Australia’s National Medicines Policy. Future
evaluations will be required to determine whether this balance is maintained.
136
References
1. Industry Commission. The pharmaceutical industry. Volume 1: the report. Melbourne:
Australian Government Publishing Service; 1996.
2. Australian Government Department of Health and Ageing. Schedule of pharmaceutical
benefits for approved pharmacists and medical practitioners. Operative from 1 March
2007. Canberra: Commonwealth of Australia; 2007. Available from:
http://www.pbs.gov.au/html/healthpro/publication/archive. [Accessed 13th December
2007].
3. Commonwealth of Australia. Pharmaceutical Benefits Pricing Authority annual report
for the year ended 30 June 2006. Canberra: Department of Health and Ageing,
Pharmaceutical Evaluation Branch; 2006. Available from:
http://www.health.gov.au/internet/wcms/publishing.nsf/Content/D54934A7E75638F6CA2
572170082946F/$File/pbpa%20annual%20report%202006.pdf. [Accessed 13th December
2007].
4. Australian Divisions of General Practice Ltd. GPs call for "one switch per script" [News
Release]. 2003. Available from: http://www.adgp.com.au/client_images/5024.pdf.
[Accessed 25th October 2004]
5. Ferguson H. GP calls for restrictions on generic substitution. Australian Doctor 2006;
February 15. Available from:
http://www.australiandoctor.com.au/articles/49/0c03ce49.asp. [Accessed 13th December
2007]
6. Cain C. Think twice before accepting substitute drugs. The Advertiser. 2006 December
29; Section: Opinion. p 20.
7. Pharmacy Board of South Australia. Complaints and investigations. Pharmacy Board of
South Australia Newsletter 2004; August(10): 4. Available from:
http://www.pharmacyboard.sa.gov.au/newsletters/August%202004.pdf. [Accessed March
30th 2007]
8. Deakin G, Aufgang M. Controversies in medicine: wrestling with brand choices.
Australian Doctor 2002;September 13:42-3.
9. Pharmaceutical Health and Rational Use of Medicines (PHARM) Consumer Sub-
Committee. Consumer perspectives on managing multiple medicines. Canberra: PHARM;
2001. Available from:
http://www.health.gov.au/internet/wcms/publishing.nsf/Content/nmp-pdf-pharmconsumer-
cnt.htm. [Accessed 13th December 2007].
10. Brooker C. No simple messages on generic substitution. Pharmacy News 2003;August
21:8.
11. Peterson G. Generics: an issue of complexity and angst (letter). Pharmacy News
2003;September 18:7.
137
12. Sorensen L, Stokes J, Purdie D, Woodward M, Roberts M. Medication management at
home: medication-related risk factors associated with poor health outcomes. Age and
Ageing 2005;34:626-32.
13. Peterson G, Naunton M. Simple measures to assist with drug therapy can produce large
benefits for elderly patients. Australian Pharmacist 2002;21(5):370-3.
14. Commonwealth of Australia. National medicines policy. Canberra: Commonwealth
Department of Health and Aged Care; 1999.
15. World Health Organization. Guidelines for developing national drug policies. Geneva:
World Health Organization; 1988.
16. World Health Organization. How to develop and implement a national drug policy.
WHO Policy Perspectives on Medicines Issue 6, January 2003. Available from:
http://www.who.int/medicines/publications/policyperspectives/PPM No6-6pg-en.pdf.
[Accessed 6th March 2007]
17. Sloan C. A history of the Pharmaceutical Benefits Scheme 1947 - 1992. Canberra:
Australian Government Publishing Service; 1995.
18. Sansom L. The Australian national medicinal drug policy. Journal of Quality in
Clinical Practice 1999;19:31-5.
19. Commonwealth of Australia. The national strategy for quality use of medicines.
Canberra: Department of Health and Ageing; 2002.
20. Hunter T. Some thoughts on the Pharmaceutical Benefits Scheme. Australian Journal
of Social Issues 1963;1(4):32-40.
21. Australian Government Department of Health and Ageing. Strengthening your PBS -
preparing for the future. 2006. Available from:
http://www.health.gov.au/internet/wcms/publishing.nsf/Content/A2F23E7630B8F9F3CA2
57227007F1EC7/$File/strengthening-your-PBS161106.pdf. [Accessed 13th March 2007]
22. Australian Government Department of Health and Aged Care. The Australian health
care system: an outline. Canberra: Department of Health and Aged Care; 2000. Available
from: http://www.health.gov.au/internet/wcms/publishing.nsf/Content/haf-ozhealth-
index/$FILE/ozhealth.pdf. [Accessed 22nd March 2007].
23. Australian Government Department of Health and Ageing. Schedule of pharmaceutical
benefits for approved pharmacists and medical practitioners. Effective 1 January 2008 - 31
January 2008. Commonwealth of Australia; 2008. Available from:
http://www.pbs.gov.au/html/healthpro/publication/archive. [Accessed 11th January 2008].
24. Therapeutic Goods Administration. Information kit. Canberra: Therapeutic Goods
Administration; 1999.
25. McEwen J. What does TGA approval of medicines mean? Australian Prescriber
2004;27(6):156-8.
138
26. Tett S. A perspective on Australia's national medicines policy. Canadian Journal of
Clinical Pharmacology 2004; 11(1): e28-e38. Available from:
http://www.cjcp.ca/pdf/Australia National MedicinesDEMO.pdf. [Accessed 13th
December 2007]
27. Harvey K, Murray M. Medicinal drug policy. In: Gardner H, editor. Politics of health.
2nd ed. Melbourne: Churchill Livingstone; 1995. p. 238-83.
28. Commonwealth Department of Health and Ageing, Therapeutic Goods Administration.
Australian code of good manufacturing practice for medicinal products. 16 August 2002.
Available from: http://www.tga.gov.au/docs/pdf/gmpcodau.pdf. [Accessed 13th December
2007]
29. Weekes L, Mackson J, Fitzgerald M, Phillips S. National Prescribing Service: creating
an implementation arm for national medicines policy. British Journal Of Clinical
Pharmacology 2005;59(1):112-6.
30. Productivity Commission. Evaluation of the pharmaceutical industry investment
program. Research report. Canberra: AusInfo; 2003.
31. Commonwealth of Australia. Management of the pharmaceuticals partnerships
program. Audit report no.46 2006-07. Canberra: Australian National Audit Office; 2007.
32. Commonwealth of Australia. Fact sheet: Pharmaceuticals Partnerships Program (P3).
Available from: http://ausindustry.gov.au/content/content.cfm?ObjectID=229AEF11-
7DC5-4DFA-B95F72C194FCA90D. [Accessed April 24th 2007].
33. Boersma C, Klok R, Bos J, Naunton M, van den Berg P, de Jong-van den Berg L, et al.
Drug costs developments after patent expiry of enalapril, fluoxetine and ranitidine. A study
conducted for the Netherlands. Applied Health Economics and Health Policy
2005;4(3):191 - 6.
34. OECD. Health at a glance. OECD indicators 2005. Paris: OECD publishing; 2005.
35. Freemantle N, Bloor K. Lessons from international experience in controlling
pharmaceutical expenditure. 1: influencing patients. British Medical Journal
1996;312:1469-71.
36. Mrazek M. Comparative approaches to pharmaceutical price regulation in the
European union. Croatian Medical Journal 2002;43(4):453-61.
37. Harvey K, Kalanj K, Stevanovic R. Croatian pharmaceutical sector reform project:
rational drug use. Croatian Medical Journal 2004;45(5):611-9.
38. Ess S, Schneeweiss S, Szucs T. European healthcare policies for controlling drug
expenditure. Pharmacoeconomics 2003;21(2):89-103.
39. Doran E, Robertson J, Henry D. Moral hazard and prescription medicine use in
Australia - the patient perspective. Social Science and Medicine 2005;60:1437 - 43.
139
40. Maling T, Bishop N. A New Zealand prescribing quality initiative, PreMeC - the first
10 years. International Journal of Pharmaceutical Medicine 2001;15(5):245-9.
41. Birkett D, Mitchell A, McManus P. A cost-effectiveness approach to drug subsidy and
pricing in Australia. Health Affairs 2001;20(3):104-14.
42. Donaldson C, Gerard K. Economics of health care and financing. The visible hand.
London: The Macmillan Press Ltd.; 1993.
43. Wonderling D, Gruen R, Black N. A simple model of demand. In: Black N and Raine
R, editors. Introduction to health economics. Berkshire: Open University Press; 2005. p. 37
- 55.
44. Feldstein P. Health care economics. 6th ed. New York: Thomson Delmar Learning;
2005.
45. Wonderling D, Gruen R, Black N. Supply and price determination. In: Black N and
Raine R, editors. Introduction to health economics. Berkshire: Open University Press;
2005. p. 56 - 73.
46. Hutton J, Borowitz M, Oleksy I, Luce B. The pharmaceutical industry and health
reform: lessons from Europe. Health Affairs 1994;13(3):98-111.
47. Shrank W, Young H, Ettner S, Glassman P, Asch S, Kravitz R. Do the incentives in 3-
tier pharmaceutical benefit plans operate as intended? Results from a physician leadership
survey. American Journal of Managed Care 2005;11:16-22.
48. Mullins C, Palumbo F, Saba M. Formulary tier placement for commonly prescribed
branded drugs: benchmarking and creation of a preferred placement index. American
Journal of Managed Care 2007;13(2):377-84.
49. Bloor K, Freemantle N. Lessons from international experience in controlling
pharmaceutical expenditure II: influencing doctors. British Medical Journal
1996;312:1525-7.
50. Bloor K, Maynard A, Freemantle N. Lessons from international experience in
controlling pharmaceutical expenditure III: regulating industry. British Medical Journal
1996;313:33-5.
51. Sweeny K. Price and quantity trends in the Pharmaceutical Benefits Scheme. Draft
working paper no. 14: Centre for Strategic Economic Studies, Victoria University of
Technology; August 2003. Available from: http://www.cfses.com/documents/pharma/14-
Price and Quantity Trends.PDF. [Accessed April 16th 2007].
52. Commonwealth of Australia. Pharmaceutical Benefits Pricing Authority Annual Report
for the year ended 30 June 2005. Canberra: Australian Government Department of Health
and Ageing; 2005. Available from:
http://www.health.gov.au/internet/wcms/publishing.nsf/Content/350C5B2F2D00D2C2CA
2570D7001CD22A/$File/04-05%20PBPA%20Annual%20Report.pdf. [Accessed 13th
December 2007].
140
53. Data and Modelling Section, Pharmaceutical Policy and Analysis Branch. Expenditure
and prescriptions twelve months to 30 June 2006. Canberra: Department of Health and
Ageing; 2006. Available from:
http://www.health.gov.au/internet/wcms/publishing.nsf/Content/A58720844CBFCB47CA
257218000D91C7/$File/complete.pdf. [Accessed 13th December 2007].
54. Commonwealth of Australia. Pharmaceutical Benefits Pricing Authority annual report
for the year ended 30 June 2000. Canberra: Department of Health and Ageing; 2000.
Available from:
http://www.health.gov.au/internet/wcms/publishing.nsf/Content/F7A1C4D9BE556D09CA
256F180046E18C/$File/pbparpt00.pdf. [Accessed 13th December 2007].
55. Grobler M. Economic analysis: is it working? Australian Prescriber 1999;22(3):50-1.
56. Harvey K. The Pharmaceutical Benefits Scheme under threat. Health Issues
2002;71:11-5.
57. Commonwealth of Australia. The Pharmaceutical Benefits Scheme: Options for cost
control. Canberra: Department of the Parliamentary Library; 2002.
58. Pharmaceutical Benefits Advisory Committee. Guidelines for preparing submissions to
the Pharmaceutical Benefits Advisory Committee (Version 4.0). Canberra: Department of
Health and Ageing; 2006. Available from:
http://www.health.gov.au/internet/wcms/publishing.nsf/Content/D8EBFB77AC0E7552CA
25717D000AE40B/$File/pbac_guidelines.pdf. [Accessed 13th December 2007].
59. Pharmaceutical Benefits Pricing Authority. Policies, procedures and methods used in
the pricing of pharmaceutical products. Canberra: Pharmaceutical Benefits Pricing
Authority; May 2006. Available from:
http://www.health.gov.au/internet/wcms/publishing.nsf/Content/A7B2821D76A9A622CA
256F180046E10A/$File/pbpa%20manual%20aug06.pdf. [Accessed April 12th 2007].
60. Australian Government Department of Health and Ageing, Therapeutic Goods
Administration. Australian regulatory guidelines for prescription medicines. 2004 June.
Available from: http://www.tga.gov.au/pmeds/argpm.pdf. [Accessed 14th December 2007]
61. Commonwealth Department of Health and Ageing, Therapeutic Goods Administration.
CPMP guidelines - as adopted in Australia by the TGA with amendment - note for
guidance on the investigation of bioavailability and bioequivalence. 2002. Available from:
http://www.tga.gov.au/docs/pdf/euguide/ewp/140198entga.pdf. [Accessed 14th December
2007]
62. Birkett D. Generics - equal or not? Australian Prescriber 2003;26(4):85-7.
63. Sansom L. The subsidy of pharmaceuticals in Australia: processes and challenges.
Australian Health Review 2004;28(2):194-205.
64. Ioannides-Demos L, Ibrahim J, McNeil J. Reference-based pricing schemes: Effect on
pharmaceutical expenditure, resource utilisation and health outcomes. Pharmacoeconomics
2002;20(9):577-91.
141
65. Pauly M. Medicare drug coverage and moral hazard. Health Affairs 2004;23(1):113-
22.
66. McManus P. Drug utilisation. Medical Journal of Australia 1993;158:724.
67. Donnelly N, McManus P, Dudley J, Hall W. Impact of increasing the re-supply interval
on the seasonality of subsidised prescription use in Australia. Australian and New Zealand
Journal of Public Health 2000;24(6):603-6.
68. McManus P, Donnelly N, Henry D, Hall W, Primrose J, Lindner J. Prescription drug
utilisation following patient co-payment changes in Australia. Pharmacoepidemiology and
Drug Safety 1996;5:385-92.
69. Ramis Corporation Pty. Ltd. Pharmaceutical benefits study. A report on qualitative and
quantitative research conducted among holders of pensioner health benefits cards and
health benefits cards and their carers. Canberra: Department of Health Housing and
Community Services, Pharmaceutical Benefits Branch; 1993.
70. Doran E, Robertson J, Rolfe I, Henry D. Patient co-payments and use of prescription
medicines. Australian and New Zealand Journal of Public Health 2004;28(1):62 - 7.
71. Gibson T, Ozminkowski R, Goetzel R. The effects of prescription drug cost sharing: A
review of the evidence. American Journal of Managed Care 2005;11:730 - 40.
72. Goldman D, Joyce G, Zheng Y. Prescription drug cost sharing. Associations with
medication and medical utilization and spending and health. Journal of the American
Medical Association 2007;298(1):61-9.
73. Briesacher B, Gurwitz J, Soumerai S. Patients at-risk for cost-related medication
nonadherence: A review of the literature. Journal of General Internal Medicine
2007;22(6):864-71.
74. Lexchin J, Grootendorst P. Effects of prescription drug user fees on drug and health
services use and on health status in vulnerable populations: A systematic review of the
evidence. International Journal of Health Services 2004;34(1):101-22.
75. McManus P, Birkett D, Dudley J, Stevens A. Impact of the minimum pricing policy
and introduction of brand (generic) substitution into the Pharmaceutical Benefits Scheme
in Australia. Pharmacoepidemiology and Drug Safety 2001;10:295-300.
76. Silberstein N. Generic prescribing or labelling [letter]. Australian Prescriber
1994;17(4):81.
77. Gould-Hurst P. Quality use of generic medicines [letter]. Australian Prescriber
2005;28(2):30.
78. George C. Generic overdosage (letter). Medical Journal of Australia 1988;148:266-7.
79. Jackson J. Analysis of the impact of public hospital pharmaceutical reforms on
discharge medication supply. Australian Journal of Hospital Pharmacy 2001;31(4):295-9.
142
80. Australian Pensioners' and Superannuants' Federation. The impact of the minimum
pricing policy on older consumers - report to the Department of Health, Housing, Local
Government and Community Services Pharmaceutical Benefits Branch. Surry Hills:
Australian Pensioners' and Superannuants' Federation; 1993.
81. Hassali M, Kong D, Stewart K. Generic medicines: perceptions of consumers in
Melbourne, Australia. International Journal of Pharmacy Practice 2005;13:257 - 64.
82. Hassali M, Kong D, Stewart K. Generic medicines: perceptions of community
pharmacists in Melbourne, Australia. Journal of Pharmaceutical Finance, Economics and
Policy 2005;14(3):27-45.
83. Saunders C. Ticked box holds weight. Australian Doctor 2000;January 14:29-30.
84. Day R, Bell K, Rose D. The pros and cons of generic prescribing. Australian Medicine
1993;1 February:16-7.
85. Getting used to generics. Sydney Morning Herald. 2003 January 14; Section: News and
Features. p 14.
86. Metherell M. Double dose fears over drug changes. Sydney Morning Herald. 2003
January 13; Section: News and Features. p 1.
87. Peard M. Substitution issue under fire. Retail Pharmacy 2000;March:5-8.
88. Messenger A. Substitution bitter pill to swallow for some. Australian Doctor 1995;June
16:4.
89. Hassali M, Kong D, Stewart K. Generic medicines: perceptions of general practitioners
in Melbourne, Australia. Journal of Generic Medicines 2006;3(3):214 - 25.
90. AMA Federal Secretariat. AMA brand substitution survey. 2006. Available from:
http://www.ama.com.au/web.nsf/doc/WEEN-
6PX29R/$file/Brand_substitution_survey_results_May_2006.pdf. [Accessed 14th
December 2007]
91. AMA wrong on brand substitution. Australian Pharmacist 2006;25(7):506.
92. Hall A. The facts on brand substitution. AMA wrong on brand substitution [media
release]. June 1st 2006. Available from: http://www.psa.org.au/site.php?id=1054.
[Accessed July 13 2007]
93. Simoens S, De Coster S. Sustaining generic medicines markets in Europe. Belgium:
Research Centre for Pharmaceutical Care and Pharmaco-economics, Katholieke
Universiteit Leuven; 2006.
94. Andersson K, Sonesson C, Petzold M, Carlsten A, Lonnroth K. What are the obstacles
to generic substitution? An assessment of the behaviour of prescribers, patients and
pharmacies during the first year of generic substitution in Sweden. Pharmacoepidemiology
and Drug Safety 2005;14(5):341-8.
143
95. Druss B, Marcus S, Olfson M, Pincus H. Listening to generic Prozac: Winners, losers
and sideliners. Health Affairs 2004;23(5):210-6.
96. Jackson G. Generic prescribing - cost saving chaos [editorial]. International Journal of
Clinical Practice 2005;59(2):125.
97. Jackson G. Generic prescribing - the chaos continues. International Journal of Clinical
Practice 2005;59(6):618.
98. Klok R, Boersma C, Oosterhuis I, Visser S, de Jong-van den Berg L, Postma M.
Switch patterns before and after patent expiry of omeprazole: a case study in The
Netherlands. Alimentary Pharmacology and Therapeutics 2006;23:1595-600.
99. Datamonitor. Patent expiry impact predictor. Blockbuster product patent expiry.
Journal of Generic Medicines 2004;1(3):273-80.
100. Datamonitor. Nexium case study. Focusing a launch strategy on switching existing
prescriptions. London; 2004.
101. Shrank W, Stedman M, Ettner S, DeLapp D, Dirstine J, Brookhart M, et al. Patient,
physician, pharmacy and pharmacy benefit design factors related to generic medication
use. Journal of General Internal Medicine 2007;22(9):1298 - 304.
102. Spence M, Hui R, Chan J. Cost reduction strategies used by elderly patients with
chronic obstructive pulmonary disease to cope with a generic-only pharmacy benefit.
Journal of Managed Care Pharmacy 2006;12(5):377-82.
103. Andermann F, Duh M, Gosselin A, Paradis P. Compulsory generic switching of
antiepileptic drugs: high switchback rates to branded compounds compared to other drug
classes. Epilepsia 2007;48(3):464-9.
104. Chung S, Wang N, Hank N. Comparative retention rates and long-term tolerability of
new antiepileptic drugs. Seizure 2007;16:296-304.
105. McManus P, Mant A, Mitchell P, Dudley J. Length of therapy with selective
serotonin reuptake inhibitors and tricyclic antidepressants in Australia. Australian and New
Zealand Journal of Psychiatry 2004;38(6):450-4.
106. Deambrosis P, Saramin C, Terrazzani G, Scaldaferri L, Debetto P, Giusti P, et al.
Evaluation of the prescription and utilisation patterns of statins in an Italian local health
unit during the period 1994 - 2003. European Journal of Clinical Pharmacology
2007;63:197-203.
107. Feely M, Crawford P, Kramer G, Guberman A. Risk management in epilepsy: generic
substitution and continuity of supply. European Journal of Hospital Pharmacy Science
2005;11(4):83-7.
108. Tilyard M, Dovey S, Rosenstreich D. General practitioners' views on generic
medication and substitution. New Zealand Medical Journal 1990;103:318-20.
144
109. Kirsche M. Generics now playing pharmaceutical name game. Drug Store News
2004;August 23:64.
110. MacKay P. Is PHARMAC's sole-supply tendering policy harming the health of New
Zealanders? New Zealand Medical Journal 2005;118(1214):1433.
111. Mann S. Dihydropyridines, felodipine, and PHARMAC. New Zealand Medical
Journal 2005;118(1218):1569.
112. Donnelly N. The use of interrupted time series analysis to evaluate the impact of
Pharmaceutical Benefits Scheme policies on drug utilisation in Australia. Sydney:
University of New South Wales; 2005.
113. Australian Government Department of Health and Ageing. Schedule of
Pharmaceutical Benefits for Approved Pharmacists and Medical Practitioners. Effective
from 1 May 2001. Canberra: Australian Government Publishing Service; 2001.
114. Medicare Australia. Pharmaceutical benefits schedule item statistics. Available from:
http://www.medicareaustralia.gov.au/statistics/dyn_pbs/forms/pbs_tab1.shtml. [Accessed
February 5th 2008]
115. Schneeweiss S, Walker A, Glynn R, Maclure M, Dormuth C, Soumerai S. Outcomes
of reference pricing for angiotensin-converting-enzyme inhibitors. New England Journal of
Medicine 2002;346(11):822-9.
116. Thomas M, Mann J, Williams S. The impact of reference pricing on clinical lipid
control. New Zealand Medical Journal 1998;111(1071):292-4.
117. Schneeweiss S, Soumerai S, Maclure M, Dormuth C, Walker A, Glynn R. Clinical
and economic consequences of reference pricing for dihydropyridine calcium channel
blockers. Clinical Pharmacology and Therapeutics 2003;74(4):388-400.
118. Grootendorst P, Dolovich L, O'Brien B, Holbrook A, Levy A. Impact of reference-
based pricing of nitrates on the use and costs of anti-anginal drugs. Canadian Medical
Association Journal 2001;165(8):1011-9.
119. Marshall J, Grootendorst P, O'Brien B, Dolovich L, Holbrook A, Levy A. Impact of
reference-based pricing for histamine-2 receptor antagonists and restricted access for
proton pump inhibitors in British Columbia. Canadian Medical Association Journal
2002;166(13):1655-62.
120. Hazlet T, Blough D. Health services utilization with reference drug pricing of
histamine2 receptor antagonists in British Colombia elderly. Medical Care 2002;40(8):640-
9.
121. Schneeweiss S, Avorn J. A review of uses of health care utilization databases for
epidemiologic research on therapeutics. Journal of Clinical Epidemiology 2005;58:323-37.
145
122. Schneeweiss S, Maclure M, Soumerai S. Prescription duration after drug copay
changes in older people: methodological aspects. Journal of the American Geriatrics
Society 2002;50(3):521-5.
123. Johnson R, Goodman M, Hornbrook M, Eldredge M. The impact of increasing patient
prescription drug cost sharing on therapeutic classes of drugs received and on the health
status of elderly HMO members. Health Services Research 1997;32(1):103-22.
124. Tamblyn R, Laprise R, Hanley J, Abrahamowicz M, Scott S, Mayo N, et al. Adverse
events associated with prescription drug cost-sharing among poor and elderly persons.
Journal of the American Medical Association 2001;285(4):421-9.
125. Dormuth C, Glynn R, Neumann P, Maclure M, Brookhart A, Schneeweiss S. Impact
of two sequential drug cost-sharing policies on the use of inhaled medications in older
patients with chronic obstructive pulmonary disease or asthma. Clinical Therapeutics
2006;28(6):964-78.
126. Schneeweiss S, Patrick A, Maclure M, Dormuth C, Glynn R. Adherence to statin
therapy under drug cost sharing in patients with and without acute myocardial infarction. A
population-based natural experiment. Circulation 2007;115:2128-35.
127. Fairman K, Motheral B, Henderson R. Retrospective, long-term follow-up study of
the effect of a three-tier prescription drug copayment system on pharmaceutical and other
medical utilization and costs. Clinical Therapeutics 2003;25(12):3147-61.
128. Pilote L, Beck C, Richard H, Eisenberg M. The effects of cost-sharing on essential
drug prescriptions, utilisation of medical care and outcomes after acute myocardial
infarction in elderly patients. Canadian Medical Association Journal 2002;167(3):246-52.
129. Kozyrskyj A, Mustard C, Cheang M, Simons F. Income-based drug benefit policy:
impact on receipt of inhaled corticosteroid prescriptions by Manitoba children with asthma.
Canadian Medical Association Journal 2001;165(7):897-902.
130. Anis A, Guh D, Lacaille D, Marra C, Rashidi A, Li X, et al. When patients have to
pay a share of drug costs: effects on frequency of physician visits, hospital admissions and
filling of prescriptions. Canadian Medical Association Journal 2005;173(11):1335-40.
131. Hsu J, Price M, Huang J, Brand R, Fung V, Hui R, et al. Unintended consequences of
caps on Medicare drug benefits. New England Journal of Medicine 2006;354(22):2349-59.
132. Moore D, McCabe G. Introduction to the practice of statistics. Second ed. New York:
W.H. Freeman and Company; 1993.
133. Shoukri M, Pause C. Statistical methods for health sciences. Second ed. Boca Raton:
CRC Press; 1999.
134. Strom B. Study designs available for pharmacoepidemiology studies. In: Strom B,
editor. Pharmacoepidemiology. 3rd ed. West Sussex: John Wiley & Sons; 2000. p. 17-29.
146
135. Greenhalgh T. How to read a paper. The basics of evidence-based medicine. Third ed.
Oxford: Blackwell publishing; 2006.
136. Grimes D, Schulz K. Cohort studies: marching towards outcomes. Lancet
2002;359:341-5.
137. Guyatt G, Rennie D, editors. Users' guide to the medical literature. Essentials of
evidence-based clinical practice. Chicago: AMA Press; 2002.
138. Australian Government Department of Veteran's Affairs. DVA facts IS160.
Concessions and benefits. Overview of cards available to veterans and their dependants.
2007. Available from: http://www.dva.gov.au/factsheets/default.htm (select fact sheet
IS160 from the numeric index). [Accessed 14th December 2007]
139. Roughead E, Pratt N, Peck R, Gilbert A. Improving medication safety: influence of a
patient-specific prescriber feedback program on rate of medication reviews performed by
Australian general practitioners. Pharmacoepidemiology and Drug Safety 2007;16:797-
803.
140. Australian Government Department of Veterans' Affairs. Treatment population
statistics. Quarterly report - March 2007. Data extract as at 30 March 2007. 2007.
Available from:
http://www.dva.gov.au/media/publicat/Statistics/statistics/tpop/TpopMar07.pdf. [Accessed
June 7th 2007]
141. Australian Government Department of Veteran's Affairs. Australian veterans and war
widows. Your lives, your needs 2003. Canberra: Department of Veteran's Affairs; 2004.
142. Australian Bureau of Statistics. Population by age and sex, Australian states and
territories. ABS cat. no. 3201.0. 2006. Available from:
http://www.ausstats.abs.gov.au/ausstats/subscriber.nsf/0/8C8443D8A7E676EECA2572420
01B232D/$File/32010_jun%202006.pdf. [Accessed 14th December 2007]
143. Australian Institute of Health and Welfare (AIHW). Health care usage and costs. A
comparison of veterans and war widows and widowers with the rest of the community.
Cat. no. PHE 42. Canberra: AIHW; 2002.
144. Roughead E, McDermott B, Gilbert A. Antidepressants: prevalence of duplicate
therapy and avoidable drug interactions in Australian veterans. Australian and New
Zealand Journal of Psychiatry 2007;41(4):366-70.
145. Roughead E, Anderson B, Gilbert A. Potentially inappropriate prescribing among
Australian veterans and war widows/widowers. Internal Medicine Journal 2007;37(6):402-
5.
146. Edmonds D, Dumbrell D, Primrose J, McManus P, Birkett D, Demirian V.
Development of an Australian drug utilisation database. A report from the Drug Utilisation
Subcommittee of the Pharmaceutical Benefits Advisory Committee. Pharmacoeconomics
1993;3(6):427-32.
147
147. Hurley S, McNeil J, Berbatis C. Sources of Australian pharmacoepidemiology data.
Community Health Studies 1988;12(1):82-96.
148. Lim W, Woodward M. Improving medication outcomes in older people. Australian
Journal of Hospital Pharmacy 1999;29(2):103-7.
149. Lim W, Mason J. The importance of home visits: a case of extreme polypharmacy.
Australasian Journal on Ageing 2000;19(2):94-5.
150. Australian Government Department of Health and Ageing. Schedule of
Pharmaceutical Benefits for Approved Pharmacists and Medical Practitioners. Effective
from 1 May 2004. Canberra: Australian Government Publishing Service; 2004.
151. Australian Government Department of Health and Ageing. Schedule of
Pharmaceutical Benefits for Approved Pharmacists and Medical Practitioners. Effective
from 1 April 2005. Canberra: Australian Government Publishing Service; 2005.
152. Australian Government Department of Health and Ageing. Schedule of
Pharmaceutical Benefits for Approved Pharmacists and Medical Practitioners. Effective
from 1 August 2005. Canberra: Australian Government Publishing Service; 2005.
153. Pharmaceutical Society of Australia. Guidelines for pharmacists on PBS brand
substitution. Australian Pharmacist 2004;23(9):668.
154. Australian Pharmaceutical Advisory Council. Guiding principles to achieve continuity
in medication management. Canberra: APAC; 2005.
155. Strom B. Sample size considerations for pharmacoepidemiology studies. In: Strom B
and Kimmel S, editors. Textbook of pharmacoepidemiology. West Sussex: John Wiley &
Sons, Ltd.; 2006. p. 25-33.
156. Dailey G, Kim M, Lian J. Patient compliance and persistence with antihyperglycemic
drug regimens: Evaluation of a Medicaid patient population with type 2 diabetes mellitus.
Clinical Therapeutics 2001;23(8):1311-20.
157. van Soest E, Siersema P, Dieleman J, Sturkenboom M, Kuipers E. Persistence and
adherence to proton pump inhibitors in daily clinical practice. Alimentary Pharmacology
and Therapeutics 2006;24(2):377-85.
158. Bourgault C, Senecal M, Brisson M, Marentette M, Gregoire J-P. Persistence and
discontinuation patterns of antihypertensive therapy among newly treated patients: a
population-based study. Journal of Human Hypertension 2005;19(8):607-13.
159. Australian Government Department of Veterans' Affairs. Treatment population
statistics. Quarterly report - June 2007. Data extract as at 6 July 2007. 2007. Available
from: http://www.dva.gov.au/media/publicat/Statistics/docs/TpopJun07.pdf. [Accessed
14th December 2007]
160. Australian Government Department of Health and Ageing. Schedule of
pharmaceutical benefits for approved pharmacists and medical practitioners. Operative
148
from 1 November 2007. Commonwealth of Australia; 2007. Available from:
http://www.pbs.gov.au/html/healthpro/publication/archive. [Accessed 14th December
2007].
161. King M, Purdie D, Roberts M. Matching prescription claims with medication data for
nursing home residents: Implications for prescriber feedback, drug utilisation studies and
selection of prescription claims database. Journal of Clinical Epidemiology 2001;54:202-9.
162. Australian Bureau of Statistics. Technical paper. Census of population and housing:
Socio-economic indexes for area's (SEIFA) Australia 2001. Cat. No. 2039.0.55.001.
Canberra: ABS; 2004.
163. Australian Bureau of Statistics. Information paper: census of population and housing.
Socio-economic indexes for areas, Australia, 2001. ABS cat. no. 2039.0. Canberra: ABS;
2003.
164. Australian Institute of Health and Welfare. Rural, regional and remote health. A guide
to remoteness classifications. AIHW catalogue number PHE 53. Canberra: AIHW; 2004.
165. Sloan K, Sales A, Liu C, Fishman P, Nichol P, Suzuki N, et al. Construction and
characteristics of the RxRisk-V. A VA-adapted pharmacy-based case-mix instrument.
Medical Care 2003;41(6):761-74.
166. Fishman P, Goodman M, Hornbrook M, Meenan R, Bachman D, O'Keefe Rosetti M.
Risk adjustment using automated ambulatory pharmacy data. The RxRisk model. Medical
Care 2003;41(1):84-99.
167. Roughead E, Gilbert A, Primrose J, Sansom L. Drug-related hospital admissions: a
review of Australian studies published 1988-1996. Medical Journal of Australia
1998;168:405-8.
168. Green J, Hawley J, Rask K. Is the number of prescribing physicians an independent
risk factor for adverse drug events in an elderly outpatient population? American Journal
of Geriatric Pharmacotherapy 2007;5(1):31-9.
169. Quality Medication Care Pty. Ltd. Effectiveness and cost effectiveness of dose
administration aids (DAAs). St. Lucia: University of Queensland; 2004.
170. Mott D, Cline R. Exploring generic drug use behaviour: The role of prescribers and
pharmacists in the opportunity for generic drug use and generic substitution. Medical Care
2002;40(8):662-74.
171. Mamdani M, Tu K, Austin P, Alter D. Influence of socioeconomic status on drug
selection for the elderly in Canada. Annals of Pharmacotherapy 2002;36(5):804-8.
172. Federman A, Halm E, Zhu C, Hochman T, Siu A. Association of income and
prescription drug coverage with generic medication use among older adults with
hypertension. American Journal of Managed Care 2006;12(10):611-8.
149
173. Australian Government Department of Health and Ageing. Schedule of
pharmaceutical benefits for approved pharmacists and medical practitioners. Effective
from 1 April 2006. Canberra: National Capital Printing; 2006.
174. Lee T. A switch in time saves more than nine. Harvard Heart Letter 2006;16(9):1-2.
175. Maxwell A. Generic drug utilization in state Medicaid programs. Office of Evaluation
and Inspections: Drugs and Drug Industry reports 2006. Available from:
http://oig.hhs.gov/oei/reports/oei-05-05-00360.pdf. [Accessed 28th November 2007]
176. Commonwealth Department of Health and Aged Care. Schedule of pharmaceutical
benefits for approved pharmacists and medical practitioners. Effective from 1 August
2000. Canberra: J.S. McMillan Printing Group; 2000.
177. Pharmaceutical Health and Rational Use of Medicines Committee, Australian
Pharmaceutical Advisory Council. Quality use of medicines: - A decade of research,
development and service activity 1991 - 2001. 2001. Available from:
http://www.health.gov.au/internet/wcms/publishing.nsf/Content/nmp-pdf-qumresearch-
cnt.htm/$FILE/qumresearch.pdf. [Accessed October 22nd 2007]
178. Australian Pharmaceutical Advisory Council. Outcome statement of the APAC
meeting 5-6 June 2003. Available from:
http://www.health.gov.au/internet/wcms/publishing.nsf/Content/4C818AC21B1194DCCA
256F180046876F/$File/apac5jun.pdf. [Accessed October 23rd 2007]
179. Australian Pharmaceutical Advisory Council. Outcome statement of the APAC
meeting 11-12 November 2004. Available from:
http://www.health.gov.au/internet/wcms/publishing.nsf/Content/nmp-pdf-apac11nov-
cnt.htm/$FILE/apac11nov.pdf. [Accessed October 23rd 2007]
180. Australian Pharmaceutical Advisory Council. Outcome statement of the APAC
meeting 16-17 November 2005. Available from:
http://www.health.gov.au/internet/wcms/publishing.nsf/Content/14D0847B91C3FB43CA2
570FE00811419/$File/APAC31%20Outcome%20Statement.pdf. [Accessed October 23rd
2007]
181. Australian Pharmaceutical Advisory Council. Outcome statement of the APAC
meeting 25 May 2006. Available from:
http://www.health.gov.au/internet/wcms/publishing.nsf/Content/4D4C969F7025FB67CA2
571AE0083099E/$File/apac outcomestatement 25may06.pdf. [Accessed October 23rd
2007]
182. National Prescribing Service Ltd. Annual report 2006-07. Available from:
http://www.nps.org.au/resources/content/nps_annual_report_2007.pdf. [Accessed 12th
December 2007]
183. Pharmaceutical Society of Australia. Professional practice standards, version 3.
Canberra: Pharmaceutical Society of Australia; 2006.
150
184. SHPA Committee of Specialty Practice in Clinical Pharmacy. SHPA standards of
practice for clinical pharmacy. Journal of Pharmacy Practice and Research
2005;35(2):122-46.
185. The Society of Hospital Pharmacists of Australia Committee of Specialty Practice in
Rehabilitation. SHPA standards of practice for the community liaison pharmacist. Journal
of Pharmacy Practice and Research 1996;26(5):570-2.
186. Searles A, Jefferys S, Doran E, Henry D. Reference pricing, generic drugs and
proposed changes to the Pharmaceutical Benefits Scheme. Medical Journal of Australia
2007;187(4):236-9.
187. Faunce T, Lofgren H. Drug price reforms: the new F1-F2 bifurcation. Australian
Prescriber 2007;30(6):138-40.
151
APPENDIX 1.1 – PUBLICATION ARISING FROM THIS THESIS
Kalisch LM, Roughead EE, Gilbert AL. Do pharmacists
adhere to brand substitution guidelines? The example of
simvastatin. Journal of Pharmacy Practice and Research
2007; 37(4): 292-294.
152
APPENDIX 1.2 – PUBLICATION ARISING FROM THIS THESIS
Kalisch LM, Roughead EE, Gilbert AL. Pharmaceutical
brand substitution in Australia – are there multiple
switches per prescription? Australian and New Zealand
Journal of Public Health 2007; 31(4): 348 – 52.
156
APPENDIX 1.3 – PUBLICATION ARISING FROM THIS THESIS
Kalisch LM, Roughead EE, Gilbert AL. Brand substitution
or multiple switches per patient? An analysis of
pharmaceutical brand substitution in Australia.
Pharmacoepidemiology and Drug Safety 2008; 17 (6): 620
– 25.
162
APPENDIX 4.1
Department of Veterans’ Affairs administrative claims
datasets
Department of Veterans Affairs’ administrative data has been used for the present research.
In the following tables, variables contained in the datasets which are relevant to the present
research are described.
Personal data (DVA client file)
Variable
Description
A unique identifier for a patient. Patient identifiers from the DVA
Project Client Id
dataset were re-coded for this research to prevent identification of
individuals.
Age
Age of the patient.
Age Group
Patient age group.
Gender
Patient gender.
Death indicator
Indicates whether or not the veteran was alive on a given date.
Date of birth
Patient date of birth.
Date of death
Patient date of death.
The patient’s level of entitlement to subsidised health care from DVA.
Card status
There are three different types of DVA card with varying levels of
entitlement: Gold, orange and white cards.
Post Code
The postcode of residence for a patient, on a given date.
Indicates whether the patient lived independently or in a residential
Residential status aged care facility on a defined date.
169
Pharmacy data
Variable
Description
A unique identifier for a patient. Patient identifiers from the DVA
Project Client Id
dataset were re-coded for this research to prevent identification of
individuals.
PBS/RPBS
The PBS or RPBS item code identifying the medicine dispensed.
code(s)
Date of supply
The date the medicine was dispensed.
Authority
Indicates whether or not the medicine was supplied on an authority
indicator
prescription.
Quantity
Indicates the quantity of medicine dispensed.
Indicates the manufacturer of the product dispensed, which is different
for each product of a medicine. The Schedule of Pharmaceutical
Manufacturer
Benefits lists the manufacturer codes for each brand or generic product
code
of a medicine. Therefore, the manufacturer code can be used to identify
the brand or generic product dispensed.
Repeat/Original
Indicates whether an original or a repeat prescription was used at that
indicator
dispensing.
Indicates the number of times medicine has been dispensed previously
Previous
on that prescription (e.g. 0 – original dispensing, 1 – first repeat
supplies
dispensing etc.).
Indicates whether or not the prescription was dispensed under
Regulation 24 provisions. If a prescription is endorsed “Regulation 24”
Reg 24 Indicator
by the prescriber, the original supply and all repeats may be dispensed
at one time.
Date of
Indicates the date the prescription was written by the doctor.
prescription
A unique identifier for a prescriber, which indicates the prescriber who
Project
wrote the prescription used at that dispensing. Prescriber identifiers
prescriber ID
from the DVA dataset were re-coded for this research to prevent
identification of individuals.
Number of
Indicates the number of repeats authorised by the prescriber on the
repeats
original prescription.
authorised
A unique identifier for a pharmacy, which indicates the pharmacy
Project
where the medicine was dispensed. Pharmacy identifiers from the DVA
pharmacy ID
dataset were re-coded for this research to prevent identification of
individuals.
Medication
Indicates the type of medicines review conducted.
review type
Medication
Indicates the date on which a medicines review was held.
review date
170
Private hospital data
Variable
Description
A unique identifier for a patient. Patient identifiers from the DVA
Project Client Id
dataset were re-coded for this research to prevent identification of
individuals.
Admission date
Indicates the date a patient was admitted to a private hospital.
Separation date
Indicates the date a patient was discharged from a private hospital.
Medical and Allied Health data
Variable
Description
A unique identifier for a patient. Patient identifiers from the DVA
Project Client Id
dataset were re-coded for this research to prevent identification of
individuals.
Indicates the date a patient was admitted to a residential aged care
Admission date
facility.
Indicates the date a patient was discharged from a residential aged care
Discharge date
facility.
171

Seventy five million prescription records are stored within the
b
a
T
e
l 1.
m
o
C
a
p ris n
o of mul i
t
e
l
p
w
s i h
c
t
r
e s
d
n
a
n n
o - w
s i c
t
r
e
h s
pharmacy dataset. A client file is also maintained by the DVA,
M l
u i
t p e
l
which includes patient age and gender. We identified all
w
s
t
i ch r
e s
N n
o
w
s
-
t
i ch r
e s
Ra e
t ra i
t o
pharmacy claims for simvastatin dispensed between 1
(n = 4 0
2
)
5
(n = 5
2
3
6
)
3
9
( 5
I
C
%
*
)
November 2002 and 28 February 2006. Each claim record
P e
r scr b
i e s
r
1 9
. 3 ±1 0
. 7
1 3
. 7 ±0 6
. 3
1. 1
4
( 3
.
1
-
8
4
.
1
)
5
includes a patient identifier, the date of supply, strength of
i
D s e
p n i
s ng
5
.
2 6 ± .
1 61
1 4
. 4 ± .
0 8
8
1 7
. 7 ( 7
.
1
-
3
8
.
1
)
1
simvastatin dispensed, manufacturer code (indicating the brand
pha
a
m
r
cies
or generic product supplied), prescriber identifier, date of
r
O
n
i
g
i
al
.
4 54
1
± .65
2 8
. 4
1
± . 6
4
1 6
. 0 ( 5
.
1
-
8
6
.
1
)
3
prescription, dispensing pharmacy identifier and whether an
pr s
e r
c p
i ti n
o s
original or a repeat prescription was dispensed.
Switches were identified if a patient received different
i
D s e
p n i
s n s
g
.
5
1 17 ± .
3 09
9 4
. 8 ±5 8
. 4
1 6
. 0 ( 5
.
1
-
9
6
.
1
)
1
brand or generic products of the same strength of simvastatin
*p < 0 0
0
.
01 o
f r all co p
m
r
a iso s
n
at consecutive dispensings, no more than 60 days apart. The
60-day interval was calculated from the data and represents
b
a
T
le .
2
o
C m a
p r s
i n
o of mul i
t l
p e w
s
t
i h
c ers and a
p i
t n
e s
t
i
w th
the 95th percentile for time between prescription refills. If the
one or t o
w s i
w c
t
e
h s
manufacturer code was not recorded for a claim we assumed
Multip e
l
a
P i
t n
e ts
i
w th
that it was the same as the previous supply. Of the 1.4 million
swit h
c e s
r
1 or 2 s i
w t h
c es
R e
t
a
r i
t
a o
claims identified for this study, the manufacturer code was
(n = 4 0
2 5)
(n = 8
1 33 )
9
( 5
9 % C )
I *
missing for only 4%.
e
r
P
c
s i
r e
b s
r
.
1 3
9
1
± .07
.
1 61 ± 8
.
0 0
1. 0
2
(1.1 1
-
7
2
.
)
3
For switches identified after 1 November 2004 we
i
D p
s
n
e sing
.
2 6
5
1
± 6
. 1
1 6
. 9
1
± 0
. 4
.
1 1
5
(1. 8
4 1
- 5
. 4)
determined whether the same or a different prescription was
h
p ar a
m i
c es
used at consecutive dispensings. The prescription was identified
by the date of prescription and the prescriber identifier. Doctors
i
r
O gi
l
a
n
4 5
. 4
1
±
5
6
.
.
3 4
7
1
± 2
. 4
1 2
. 2 ( .
1 0
2 1
- 2
.
)
4
e
r
p
c
s i
r t
p io s
n
can write PBS prescriptions for simvastatin valid for up to six
supplies (original prescription plus a maximum of five repeat
Di e
p
s
nsi g
n s
15 7
1
.
.
3
±
9
0
5
.
3
1
0
3
± 8
. 1
1 1
. 2 (1.
-
1
1 1.
)
3
1
dispensings); and can only write one original prescription for
p
* < 0 0
.
0
0 1 for all o
c
p
m
r
a s
i n
o s
each simvastatin strength per patient per day.8 Therefore, we
considered that a patient had repeats of the same prescription
Fifty-three per cent of patients who received simvastatin
dispensed consecutively if consecutive claims showed the same
post-generic availability did not switch products. Thirty-eight
prescriber identifier and date of prescribing, and were for the
per cent of patients had only one or two substitutions, while
same strength of simvastatin.
9% were multiple switchers. Multiple switchers were likely to
The rate for the supply of different products (switching) at
have more prescribers, more dispensing pharmacies, more
consecutive dispensings was calculated per 1000 prescriptions
original prescriptions and more simvastatin dispensings than
dispensed each month. The rates pre- and post-generic
non-switchers and patients who had only one or two switches
availability were compared using negative binomial regression
(Tables 1,2).
analysis.
Patients were categorised as non-switchers, those with
DISCUSSION
only one or two switches and those with three or more switches
Results of this study suggest that, in the case of simvastatin, the
post-generics (‘multiple switchers’) for subgroup analyses.
brand substitution policy is being implemented primarily as
Differences in the number of simvastatin prescribers, dispensing
intended. Switching was 22 times more likely to occur post-
pharmacies, dispensings and number of prescriptions used were
generic availability compared to pre-generics; however, the
compared between groups using Poisson regression. All data
increase was largely due to patients switching once to a new
were analysed using SAS version 9.1 (SAS Institute).
generic product, rather than patients being switched multiple
times. In the 16-month study period, 91% of patients switched
RESULTS
twice or less. These results add to previous research where it
Between 1 November 2004 and 28 February 2006, 48 177
was shown that in a three-month period after introduction of
patients received simvastatin. Fifty-six per cent (n = 27 142)
brand premiums to ranitidine and fluoxetine, 8% and 39% of
were male and patients received an average of 11.5 ± 5.4
patients, respectively, switched to cheaper products.9 Our
dispensings. There were 39 786 switches identified post-
results demonstrate that substitution is sustained for longer
generics. For 64% of these switches (n = 25 311), different
periods after generics are introduced. The size of the premium
prescriptions were dispensed consecutively.
influenced switching in the earlier study
the ranitidine brand
Prior to generic availability, different products were
premium was only 71c compared to $5.06 for fluoxetine.9 In
supplied consecutively for 3.6 out of every 1000 prescriptions
the present study, a brand premium comparable to that for
dispensed. When the first simvastatin generic was introduced
ranitidine applied to simvastatin (70c), and over 16 months of
in November 2004 the rate of switching increased; to an average
follow-up 47% of simvastatin patients had products substituted.
rate of 78.2 switches per 1000 prescriptions dispensed from
One of the rules governing brand substitution states that
February 2005 to February 2006 (Figure 1). Switches were
products may only be substituted if they are bioequivalent.1
22 times more likely to occur post-generics than pre-generics
The results of this study suggest that pharmacists adhere to this
(rate ratio 21.91; 95%CI 20.01 24.00; p < 0.001).
rule and rarely substitute products that have not been designated
From February to July 2005 when four simvastatin
bioequivalent. Prior to introduction of the first simvastatin
products were available, the rate of switching was 79.0 switches
generic, different products were supplied at consecutive
per 1000 prescriptions dispensed. From August 2005 onwards,
dispensings for only 3.6 out of every 1000 dispensings. A high
when 10 simvastatin products were available, the rate of
switching rate pre-generic availability may have suggested that
switching was no higher; 77.5 switches per 1000 prescriptions
pharmacists substituted products which had not been shown
dispensed (RR 1.02; 95%CI 0.998 1.041; p = 0.0664).
to be bioequivalent. The low pre-generics switching rate
suggests that substitution is likely to have occurred only in
situations where it was permitted (e.g. when the patients usual
Journal of Pharmacy Practice and Research Volume 37, No. 4, 2007
293
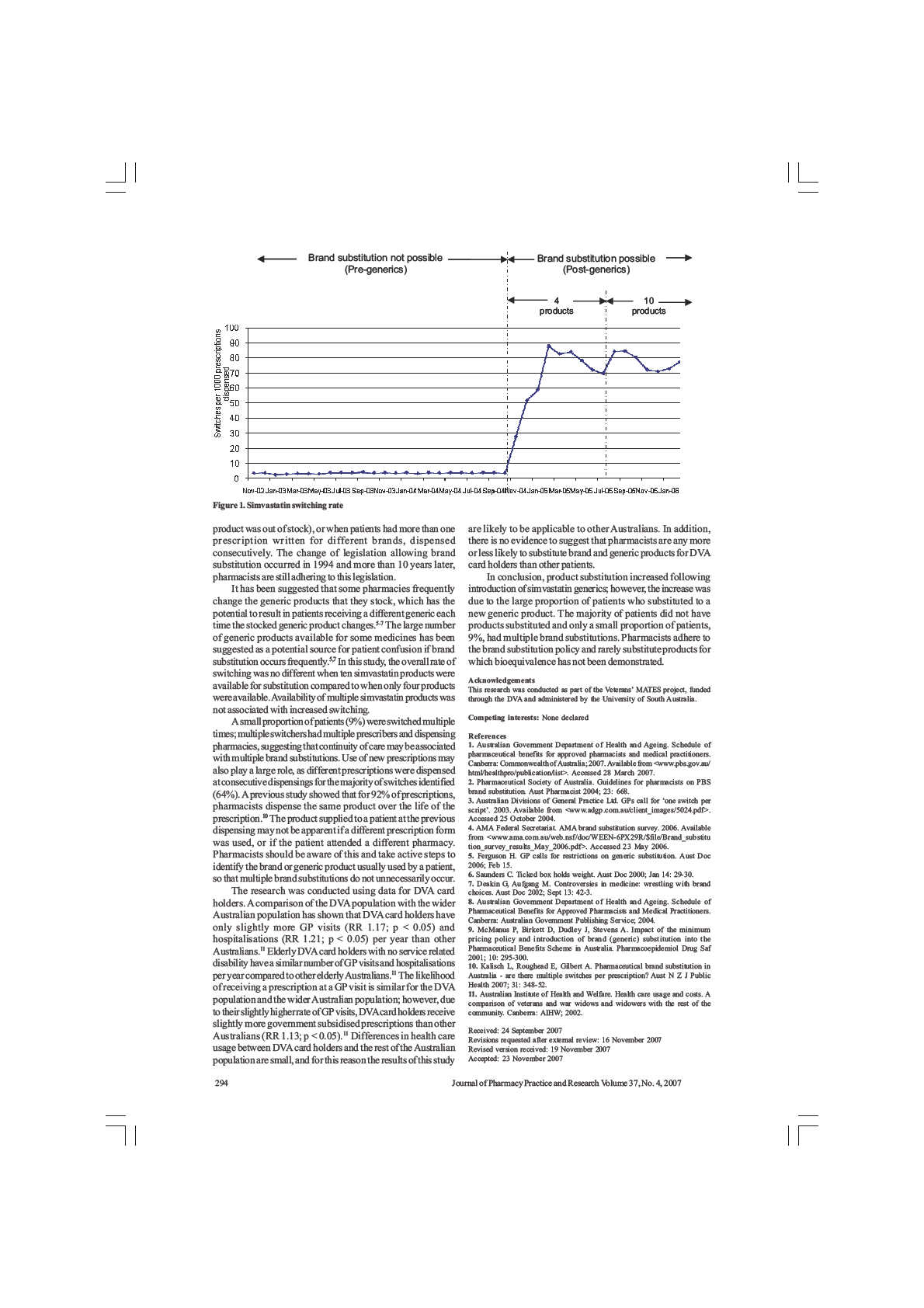
Improving Health
Article
Pharmaceutical brand substitution in Australia
– are there multiple switches per prescription?
Abstract
Lisa M. Kalisch, Elizabeth E. Roughead and Andrew L. Gilbert
Quality Use of Medicines and Pharmacy Research Centre, Sansom Institute,
Background: In Australia, brand
School of Pharmacy and Medical Sciences, University of South Australia
substitution by pharmacists has been
possible since 1994. There is no limit to the
number of substitutions per prescription.
Doctors have expressed concern that
the Pharmaceutical Health and Rational
patients may receive a different product
Use of Medicines (PHARM) committee
each time their prescription repeats are
held discussions with representatives from
dispensed, which has the potential to
In Australia, brand substitution of
Pharmaceutical Benefits Scheme (PBS)
and Repatriation Pharmaceutical
Benefits Scheme (RPBS) medicines has
more than 100 consumer groups for a range
confuse patients. It is unknown how often
been possible since December 1994, when
of chronic conditions, including patients
multiple substitutions per prescription occur.
the brand substitution policy was introduced.
from non-English-speaking backgrounds.
Objectives: We aimed to identify the
Pharmacists can dispense a brand or generic
It found that patients may not realise that
number of switches per prescription for
product other than the one prescribed
substituted brand and generic products are
a range of medicines and to determine
provided the patient agrees to the switch,
actually the same drug and problems such
the number of different brand and generic
the substituted products are bioequivalent
as double dosing (i.e. taking both products
products supplied on each prescription.
and the prescriber has not specified that
at the same time) or poor compliance may
Methods: Repatriation Pharmaceutical
substitution cannot occur. At present,
Benefits Scheme prescription claims
result.2 Concerns about multiple switches
the policy does not limit the number of
between 1 January 2001 and 28
per prescription and the potential for
substitutions that can occur on repeats of
February 2006 were identified for
patient confusion are also held by general
an individual prescription. In 2003, the
atenolol, citalopram, enalapril, metformin,
practitioners.3,4 Hassali and colleagues
omeprazole, ramipril, and simvastatin.
Australian Divisions of General Practice
interviewed a convenience sample of 10
Original prescriptions with five repeats
highlighted this and expressed concerns
Australian general practitioners (GPs) about
and all supplies dispensed were included.
about the potential for patients to receive a
their views of generic medicines.3 Some
Switches were identified if a different
different brand of medicine each time their
GPs in their sample expressed concerns
product was supplied on consecutive
prescription was dispensed, equating to six
about the potential for patient confusion
repeat dispensings.
different brands over the life of a script.1
when substitution occurred, and some
Results: 533,279 original prescriptions
They suggested that a limit of one switch
were also critical of the brand substitution
were included. 488,735 (92%) had no
per prescription should be enforced.1 Their
policy not preventing multiple switches by
switches on repeats and 37,513 (7%)
concerns about multiple substitutions per
pharmacists.3 In another Australian study
had only one switch. Only 1% of all
prescription arose from the different names
70 GPs were surveyed.4 Many of the GPs
prescriptions had more than one switch
and appearance of the various brand and
expressed concerns that generic substitution
identified on repeats, and in most cases
generic alternatives available.
had the potential to confuse patients and
only two different products were supplied.
Generic drugs in Australia are marketed
some suggested that there should be limits
None of the prescriptions had a different
under a unique trade name rather than
to the number and frequency of brand
product supplied on each dispensing.
the generic name of the drug. For drugs
Conclusion and Implications: Multiple
substitutions.4 The small sample sizes of
with multiple brand and generic products,
switches per prescription are uncommon
these studies limit the generalisability of
patients are faced with multiple different
and multiple different products are
results; however, there appears to be a
rarely supplied on repeats of the same
trade names, and in many cases the product
perception among Australian prescribers and
prescription. The rules of the brand
appearance also differs. Qualitative
consumers that brand substitution can cause
substitution policy appear to be adequate
Australian research has found that patients
confusion. The actual extent of the problem
in allowing brand choice for patients,
may become confused when products are
is unknown.
without leading to multiple switches per
substituted.2,3 Between 1998 and 2000,
Since 1997, the Pharmaceutical Society
prescription.
Key words: Generic substitution; generic
Submitted: April 2007
Accepted: June 2007
drugs, pharmaceutical policy; pharmaco-
Correspondence to:
epidemiology.
Ms Lisa Kalisch, Quality Use of Medicines and Pharmacy Research Centre, Sansom Institute,
(
Aust NZ J Public Health. 2007; 31:348-52)
School of Pharmacy and Medical Sciences, University of South Australia, GPO Box 2471,
Adelaide, SA 5001. Fax: (08) 8302 1087; e-mail: xxxx.xxxxxxx@xxxxxxxxx.xxxxx.xxx.xx
doi:10.1111/j.1753-6405.2007.00085.x
348
AUSTRALIAN AND NEW ZEALAND JOURNAL OF PUBLIC HEALTH
2007 vol. 31 no. 4
© 2007 The Authors. Journal Compilation © 2007 Public Health Association of Australia
Improving Health
Pharmaceutical brand substitution
of Australia (PSA) has had brand substitution guidelines for
The first citalopram generic became available on 1 August 2001,
pharmacists.5 These guidelines state that the health and safety
and the first simvastatin generic became available on 1 November
of the patient should be the foremost concern when substitution
2004. For these two drugs, claims were included from the date the
occurs, and that whenever possible the same product should be
first generic became available until the end of February 2006. For
supplied to patients on chronic therapy.5 The extent to which
all other study drugs, claims identified between 1 January 2001
pharmacists follow these guidelines is unknown. A survey of 312
and 28 February 2006 were included. Each claim record includes
pharmacists conducted by the PSA in mid-2006 found that 94% of
a patient identifier, date of prescription, a prescriber identifier,
respondents regularly offered brand substitution to their patients,
the number of repeats on the prescription, whether the original
and more than 70% of respondents reported that less than 20%
prescription or a repeat was dispensed, the manufacturer code
of prescriptions they dispensed had substitution prohibited by
and the date of supply. Doctors can write PBS prescriptions for
the doctor.6 Opportunities for patients to be switched are great;
each of the study drugs valid for up to six supplies (the original
however, the extent to which this translates into multiple switches
dispensing, plus a maximum of five repeat dispensings); and can
per prescription and multiple different products being supplied
only write one PBS or RPBS prescription for each strength of study
is unknown.
drug per patient per day.8 Following these rules, we determined
that when the claim record showed the same patient identifier,
prescriber identifier, date of prescribing and was for the same
Aims
strength of drug, repeats of the same prescription had been used
We aimed to identify the number of switches on the repeats of
at those dispensings. Individual prescriptions were identified and
an individual prescription and to determine how often there were
original and repeat dispensings were sorted by date of supply. The
multiple switches and multiple products supplied on the same
total number of dispensings per prescription was calculated.
prescription.
Inclusion and exclusion criteria
Original prescriptions written for five repeats with all six
Methods
supplies dispensed within the study period were included.
Study drugs
Data errors were identified if there was more than one claim for
Atenolol, citalopram, enalapril, metformin, omeprazole,
an original prescription written by the same doctor on the same
ramipril, and simvastatin were selected for study (see Table 1).
day for any patient, or if there were more claims identified for a
These drugs were chosen as they are all commonly dispensed on
prescription than allowed by the number of repeats ordered. This
the PBS and RPBS,7 cover a range of therapeutic classes, and are
led to 16,175 dispensings being excluded (0.3% of identified
generally used in the treatment of long-term conditions. All study
dispensings).
drugs have bioequivalent brand and generic alternatives available.
The manufacturer code in each claim record was used to
Strengths and formulations studied are listed in Table 1.
identify the brand or generic product dispensed. We assumed
RPBS prescription claims for all study drugs were identified.
that the original dispensing was for the brand or generic product
Table 1: Study drugs.
Study drug
Strengths and forms studied
Number of brand/
Therapeutic category and uses
generic products
Atenolol
50 mg tablets
10a
Beta blocking agent used in the treatment of
cardiovascular conditions including hypertension
Citalopram
20 mg tablets
7
SSRI antidepressant
Enalapril
5 mg, 10 mg and 20 mg tablets
11a
Angiotensin converting enzyme inhibitor used in the
treatment of cardiovascular conditions including
hypertension
Metformin
500 mg and 850 mg tablets
10a
Biguanide oral hypoglycaemic agent, used in the
treatment of type 2 diabetes
Omeprazole
20 mg tablets
2 (3 from Dec 2004
Proton pump inhibitor used in the treatment of acid-
onwards)
related gastrointestinal disorders such as
gastroesophageal reflux disease and peptic ulcer
disease.
Ramipril
1.25 mg, 2.5 mg and 5 mg tablets
2
Angiotensin converting enzyme inhibitor used in the
treatment of cardiovascular conditions including
hypertension
Simvastatin
5 mg, 10 mg, 20 mg, 40 mg
4 until August 2005,
HMG CoA-reductase inhibitor used to lower
and 80 mg tablets
then 10
cholesterol
Note:
(a) Single brand and generic products were added or deleted at certain times over the study period. Therefore, the number of products available for the majority of
the study period has been shown.
2007 vol. 31 no. 4
AUSTRALIAN AND NEW ZEALAND JOURNAL OF PUBLIC HEALTH
349
© 2007 The Authors. Journal Compilation © 2007 Public Health Association of Australia
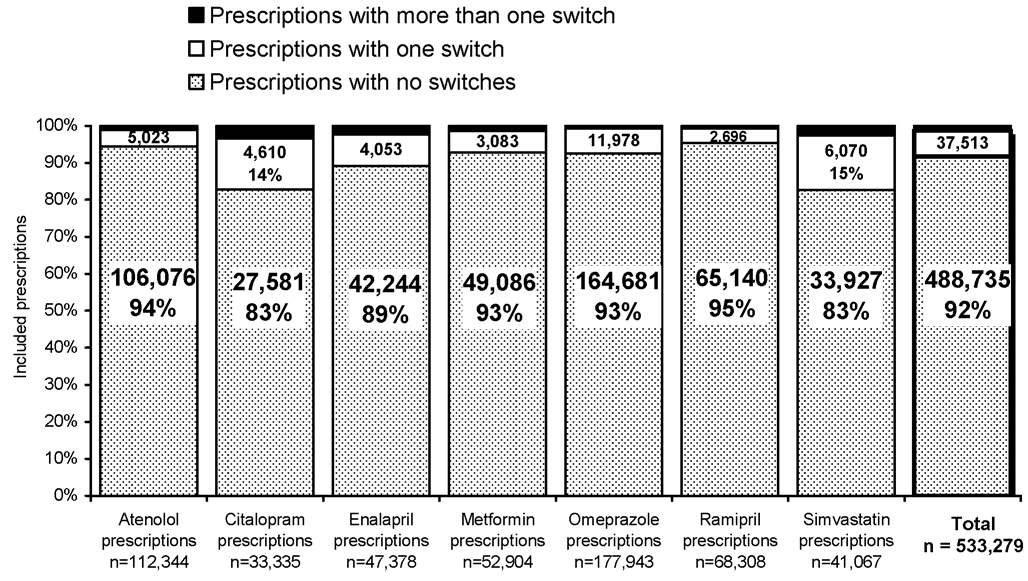 Kalisch, Roughead and Gilbert
Article
Kalisch, Roughead and Gilbert
Article
prescribed; switches were then identified if different brand or
more than one switch, and the majority of these (n=5,822) had two
generic products were supplied on consecutive repeat dispensings
switches. Only 0.2% (1,026) of all prescriptions had three switches
of the same prescription. When the manufacturer code was not
and 0.03% (161) had four switches. Of the 533,279 prescriptions
recorded for a given dispensing (3% of claims), we assumed that
studied, only 22 had a switch on every repeat dispensing (i.e. five
it was the same as the previous supply. We calculated the number
switches per prescription).
of switches per prescription and the number of different brand or
generic products dispensed over the life of the prescription. Using
Number of different products supplied
this method, we could identify a maximum of five switches per
per prescription
prescription and six different products supplied over the life of
Our definition of switching means that prescriptions with no
the prescription.
switches had the same product supplied on each repeat, and
prescriptions with one switch had two different products supplied
over the life of the prescription. In most cases, prescriptions
Results
with more than one switch also had only two different products
Overall, 533,279 original prescriptions with five repeats
dispensed over the life of the prescription (see Table 2). None of
prescribed and all supplies dispensed were identified and included
the prescriptions included in the study had a different product
in the study.
supplied with each dispensing.
Number of switches per prescription
Ninety-two per cent of prescriptions studied had no switches
Discussion
(see Figure 1). When switches were identified on repeats of the
To our knowledge, this is the first study that has identified the
same prescription, in the majority of cases there was only one
number of brand substitutions that occur on repeats of the same
switch per prescription (7% of all prescriptions). Only 1% of all
prescription for any drug. The results show that multiple switches
prescriptions identified had more than one switch.
per prescription are uncommon. For 92% of prescriptions studied,
the same product was supplied on each dispensing. If switches
Prescriptions with multiple switches
occurred, there was only one switch per prescription in the majority
Ramipril and omeprazole, the drugs with the fewest brand and
of cases (7% of prescriptions). Similarly, multiple products were
generic products, had the lowest proportions of prescriptions with
rarely supplied over the life of a prescription, even if there were
more than one switch (0.7%), while citalopram had the highest
multiple switches on that prescription. None of the prescriptions
(3%). Only 1% (n=7,031) of the 533,279 prescriptions studied had
studied had a different product supplied on each dispensing.
Figure 1: Prescriptions with and without switches.
350
AUSTRALIAN AND NEW ZEALAND JOURNAL OF PUBLIC HEALTH
2007 vol. 31 no. 4
© 2007 The Authors. Journal Compilation © 2007 Public Health Association of Australia
Improving Health
Pharmaceutical brand substitution
Pharmacists appear to consistently supply the same product over
drugs with the fewest products available (ramipril and omeprazole)
the life of most prescriptions, which is in accordance with the PSA
had the lowest proportion of prescriptions with multiple switches
guidelines for brand substitution.
(0.7%), drugs with multiple products available also had a very
The rules of the brand substitution policy do not limit the
small proportion of prescriptions with multiple switches. Only
number of switches per prescription, which led the Australian
3% of citalopram prescriptions, 2% of enalapril and simvastatin
Divisions of General Practice (now known as the Australian
prescriptions and 1% of atenolol and metformin prescriptions
General Practice Network) to express concerns that patients may
had more than one switch, and in most cases only two different
receive a different product each time their prescription repeats
products were supplied over the life of the script. These five drugs
are filled.1 These concerns have been reflected by consumers2 and
all had seven or more products available to switch between for the
general practitioners3,4 in qualitative Australian research. Research
majority of the study period.
conducted with consumers using multiple medicines found that
Brand substitution is possible for many other PBS and RPBS
confusion from brand substitution may lead to poor compliance or
medicines in addition to the seven study drugs. The study drugs
‘double dosing’ with two different brands of the same medicine.2
were chosen because they represent a wide range of therapeutic
Our study is the first to quantitatively assess the number of products
classes on the PBS and are commonly dispensed on the RPBS.
supplied on repeats of the same prescription. The results show that
The number of brand and generic products available to switch
although the rules of the brand substitution policy do not prevent
between and the length of time multiple products have been
pharmacists from dispensing a different product on each repeat,
available varied among the drugs. Despite these differences, in all
this does not occur frequently.
cases the majority of prescriptions identified for each drug had no
Given the likely inconvenience that a limit of one switch per
switches, and when switches were identified in most cases there
prescription would incur, and the fact that pharmacists already
was only one switch per prescription. Simvastatin and citalopram
supply the same product on repeats of prescriptions in most cases,
had the lowest proportions of prescriptions with no switches than
it does not appear necessary to change the rules of the brand
the other medicines studied (83%). In the case of simvastatin, this
substitution policy. A limit of one switch per prescription may
difference may have been because brand substitution had only
be impractical for pharmacists and patients.9,10 Patients may have
recently become possible. Patient-related factors may also have
their repeats dispensed at more than one pharmacy. If there was a
played a role.
limit of one switch per prescription, pharmacies may need to stock
We could not tell how many prescriptions were marked “brand
every alternative in order to be able to dispense repeats in these
substitution not permitted”, so we cannot be sure of how often
situations.9 The number of brand and generic alternatives available
switching was not possible and the influence that this had on the
for some drugs means that stocking all of them is impractical for
results. However, recent research suggests that substitution is
most pharmacies.9,10 In addition, if a particular product is out of
possible for most prescriptions. A survey of Australian doctors
stock, a limit of one switch per prescription may mean that some
found that the majority of prescribers marked less than a quarter
patients cannot have their repeats dispensed if their product is
of their prescriptions “brand substitution not permitted”,11 and a
out of stock.10
survey of pharmacists supported this finding.12
We differentiated between the number of switches per
RPBS claims were studied, therefore only prescriptions
prescription and the total number of different products supplied
dispensed to Department of Veterans’ Affairs (DVA) card holders
per prescription because the study drugs had different numbers of
were represented in the analysis. There is no evidence to suggest
products available to switch between. Only two ramipril products
that pharmacists are more or less likely to substitute brand and
were available, however a patient could potentially alternate
generic products for DVA card holders than other patients. The
between each product with each repeat dispensing. Although the
medicines studied are available on both the PBS and RPBS, and
Table 2: Number of products dispensed on prescriptions with multiple switches.
Study drug
Prescriptions
Number of different brand or generic products supplied
with > one switch
2 products
3 products
4 products
5 products
6 products
Atenolol
1,245
911
323
11
0
0
Citalopram
1,144
682
436
23
3
0
Enalapril
1,081
677
375
26
3
0
Metformin
735
547
184
3
1
0
Omeprazolea
1,284
1,231
53
–
–
–
Ramiprila
472
472
–
–
–
–
Simvastatin
1,070
631
430
9
0
0
Total
7,031
5,151
1,801
72
7
0
Note:
(a) Only two ramipril products and three omeprazole products were available for substitution.
2007 vol. 31 no. 4
AUSTRALIAN AND NEW ZEALAND JOURNAL OF PUBLIC HEALTH
351
© 2007 The Authors. Journal Compilation © 2007 Public Health Association of Australia
Kalisch, Roughead and Gilbert
Article
the subsidised quantities and prices paid by PBS concession card
References
holders are the same as those for DVA card holders. It is likely
1. Australian General Practice Network [home page on the Internet]. Canberra
that the results are equally applicable to other members of the
(AUST): Australian Divisions of General Practice Ltd; 2003 [cited 2004 Oct
25].
GPs Call for “One Switch per Script”. Available from: http://www.adgp.
Australian population.
com.au/client_images/5024.pdf
Results of this study indicate that pharmacists consistently
2. Pharmaceutical Health and Rational Use of Medicines.
Consumer Perspectives
on Managing Multiple Medicines. Canberra (AUST): Consumer Sub-
supply the same product on each repeat of a prescription in the
Committee, PHARM; 2001.
majority of cases. When switches occur, there is nearly always
3. Hassali M, Kong D, Stewart K. Generic medicines: Perceptions of general
practitioners in Melbourne, Australia.
Journal of Generic Medicines.
only one switch per script. Multiple switches per prescription are
2006;3(3):214-25.
uncommon and multiple different products are rarely supplied
4. Sweet G, Wilson S.
The 3D Labels Project. Melbourne (AUST): Dandenong
District Divisions of General Practice, Pharmacy Guild of Australia; 2004.
on repeats of the same prescription. The present rules of the
5. Pharmaceutical Society of Australia. Guidelines for Pharmacists on PBS Brand
brand substitution policy appear to be adequate in allowing
Substitution.
Australian Pharmacist. 2004;23(9):668.
6. AMA wrong on brand substitution.
Australian Pharmacist. 2006;25(7):506.
brand choice for patients, without leading to multiple switches
7. Department of Health and Ageing [publications page on the Internet]. Canberra
per prescription.
(AUST): Commonwealth of Australia; 2005 [cited 2006 Apr 3].
Pharmaceutical
Benefits Pricing Authority Annual Report for the Year Ended 30 June 2005.
Available from: http://www.health.gov.au/internet/wcms/publishing.nsf/
Content/350C5B2F2D00D2C2CA2570D7001CD22A/$File/04-05%20PBP
Acknowledgements
A%20Annual%20Report.pdf
8. Department of Health and Ageing.
Schedule of Pharmaceutical Benefits for
This research was conducted as part of the Veterans’ MATES
Approved Pharmacists and Medical Practitioners. Effective from 1 April 2006.
project, funded through the Department of Veterans’ Affairs and
Canberra (AUST): Commonwealth of Australia; 2006.
9. Hall A. Proposed generic restriction not practical.
Australian Pharmacist.
administered by the University of South Australia.
2003;22(2):110.
10. Drug switch confusion debated.
Australian Journal of Pharmacy.
2003;84:76.
11. Australian Medical Association [publication page on the Internet]. Canberrra
(AUST): Federal Secretariat, AMA; 2006 [cited 2006 May 23].
AMA Brand
Substitution Survey. Available from: http://www.ama.com.au/web.nsf/doc/
WEEN-6PX29R/$file/Brand_substitution_survey_results_May_2006.pdf
12. Hall A. The Facts on Brand Substitution. AMA Wrong on Brand Substitution
[media release page on the Internet]. Canberra (AUST): Pharmaceutical
Society of Australia; 2006 June 1 [cited 2007 July 13]. Available from:
http://www.psa.org.au/site.php?id=1054.
352
AUSTRALIAN AND NEW ZEALAND JOURNAL OF PUBLIC HEALTH
2007 vol. 31 no. 4
© 2007 The Authors. Journal Compilation © 2007 Public Health Association of Australia
pharmacoepidemiology and drug safety (2008)
Published online in Wiley InterScience (www.interscience.wiley.com) DOI: 10.1002/pds.1580
ORIGINAL REPORT
Brand substitution or multiple switches per patient? An
analysis of pharmaceutical brand substitution in Australiay,z
Lisa M. Kalisch B Pharm*, Elizabeth E. Roughead B Pharm, M App Sci, PhDx
and Andrew L. Gilbert B Pharm, Dip App Psych, PhDô
Quality Use of Medicines and Pharmacy Research Centre, Sansom Institute, School of Pharmacy and Medical Sciences,
University of South Australia, Adelaide, Australia
SUMMARY
Purpose
To determine the number of times patients have brand and generic products substituted under Australia’s
Pharmaceutical Benefits Scheme (PBS) brand substitution policy.
Methods
A retrospective cohort study was conducted using Repatriation Pharmaceutical Benefits Scheme (RPBS)
pharmacy claims data. Department of Veterans’ Affairs (DVA) treatment card holders with at least two dispensings of
atenolol, citalopram, enalapril, metformin, omeprazole or ramipril between 1 January 2001 and 28 February 2006 were
included. Patients were followed from first dispensing until death, cessation or study end. The main outcome measure was the
number of substitutions per patient during follow-up. Based on this, patients were defined as non-switchers, brand
substitution or multiple switchers.
Results
Data for 160 145 patients were analysed. Overall more than 80% of patients either had no switches or demonstrated
brand substitution. For all study drugs, patients were more likely to be non-switchers than have a brand substitution (RR
range 2.6 9.4, p < 0.0001) and were more likely to be non-switchers than multiple switchers (RR range 3.2 35.9,
p < 0.0001). Patients who switched were more likely to have a brand substitution than multiple switches (RR range
1.2 3.8, p < 0.0001). Multivariate logistic regression showed greater odds of being a multiple switcher with increasing
number of prescribers and dispensing pharmacies, and increasing length of follow-up.
Conclusions
Most patients in this study did not substitute products, and those who did were more likely to demonstrate
brand substitution than have multiple switches. These results suggest that the brand substitution policy is having its intended
effect for most patients. Copyright # 2008 John Wiley & Sons, Ltd.
key words
generic substitution; pharmaceutical policy; pharmacoepidemiology; Australia
Received 9 September 2007; Accepted 26 January 2008
* Correspondence to: L. M. Kalisch, Quality Use of Medicines and Pharmacy Research Centre, Sansom Institute, School of Pharmacy and
Medical Sciences, University of South Australia, GPO Box 2471, Adelaide, SA 5001, Australia.
E-mail: xxxx.xxxxxxx@xxxxxxxxx.xxxxx.xxx.xx
yDVA reviewed the manuscript prior to submission; however, DVA did not control or influence the decision to submit the manuscript for
publication. Prescription claims data from the Repatriation Pharmaceutical Benefits Scheme (RPBS) was used for this study. DVA provided
access to the RPBS prescription claims data but had no other role in the study design, data analysis and interpretation or writing of the paper.
zApproval to conduct this research was received from the Department of Veterans’ Affairs Human Research Ethics Committee on 28 April
2006.
xAssociate Professor.
ôProfessor.
Copyright # 2008 John Wiley & Sons, Ltd.
l. m. kalisch ET AL.
INTRODUCTION
of products, showed that a significant number of
patients switched to cheaper products in the 3 months
In 1990, the minimum pricing policy was introduced
post-introduction of a brand premium.3 What remains
to
the
Pharmaceutical
Benefits
Scheme
(PBS)
unclear is how many patients have products sub-
and Repatriation Pharmaceutical Benefits Scheme
stituted over a longer period of time or how
(RPBS), the national subsidised schemes for medicine
widespread is the problem of multiple switches
supply in Australia. Since then, only the cheapest
between products. This study examined what happens
product(s) of each PBS and RPBS medicine are
in practice when patients have prescriptions for
available at the patient co-payment price.1 Patients
medicines with substitutable products dispensed from
using more expensive brands pay the price difference
community pharmacy. We aimed to identify the
between the cheapest and more expensive product in
number of times products are substituted for patients
addition to the patient co-payment, in the form of a
and the extent of multiple switches between products.
brand premium. Brand substitution was introduced in
December 1994, allowing substitution of products at
the time of dispensing provided the prescriber had not
specified that substitution could not occur.2
METHODS
The minimum pricing policy was introduced to
Study drugs
enable pharmaceutical manufacturers to set higher
prices for their products and to provide a price signal
A retrospective cohort study was conducted using
to patients;3 brand substitution enables patients to
RPBS prescription claims data. The Department of
avoid paying brand premiums.1 Ideally, patients
Veterans’ Affairs (DVA) pharmacy claims database
should remain on the same product following the
includes records for all medicines dispensed to
initial substitution; however, legislation does not
veterans subsidised under the RPBS. The eligible
prevent supply of multiple products over time to a
treatment population numbered 300 000 in December
patient. When there are multiple products for a
2006.12 Seventy five million prescription records are
medicine, patients are faced with differing trade
stored within the dataset, and a client file is also
names and in many cases different product appear-
maintained by DVA which includes gender, age and
ance. Qualitative Australian research has shown that
date of death. Each pharmacy claim record includes a
different names and appearance for the same medicine
patient identifier, the date of supply and date of
can contribute to confusion when substitution occurs.4
prescription, a prescriber identifier and dispensing
Patients may not realise that substituted products are
pharmacy identifier, the quantity of medicine supplied
actually the same medicine and double dosing (using
and a manufacturer code (indicating the brand or
both products), poor compliance or therapy cessation
generic product supplied).
may result.4 In the years since introduction of the
Atenolol, citalopram, enalapril, metformin, ome-
brand substitution policy, there have been anecdotal
prazole and ramipril were selected as study drugs
reports and opinions in the literature suggesting that
because they are frequently dispensed on the RPBS,13
patients receive multiple different products5 8 and that
cover a range of therapeutic classes and are generally
this may cause confusion.4,5,9 11 However, the actual
used in the treatment of long-term conditions. The
extent of the problem is unknown.
analysis was limited to strengths and formulations of
A previous evaluation of the minimum pricing
these medicines with two or more brand and generic
policy, which examined the initial brand substitution
products available, listed in Table 1.
Table 1.
Included patients
Atenolol
Citalopram
Enalapril
Metformin
Omeprazole
Ramipril
n ¼ 44 575
n ¼ 18 414
n ¼ 15 752
n ¼ 23 456
n ¼ 67 992
n ¼ 36 814
Strengths and forms studied
50 mg
20 mg
5, 10 and
500 and
20 mg
1.25, 2.5 and
tablets
tablets
20 mg tablets
850 mg tablets
tablets
5 mg tablets
Male patients (%)
24 506 (55)
11 189 (61)
9123 (58)
15 585 (66)
41 060 (60)
23 358 (63)
Median age 28 February 2006
82 (79–85)
82 (70–86)
83 (80–87)
81 (76–85)
82 (79–86)
83 (80–86)
(interquartile range)
Median months follow-up
11 (4–32)
7 (2–19)
16 (5–40)
9 (3–26)
10 (3–28)
8 (2–21)
(interquartile range)
Copyright # 2008 John Wiley & Sons, Ltd.
Pharmacoepidemiology and Drug Safety, (2008)
DOI: 10.1002/pds
PHARMACEUTICAL BRAND
SUBSTITUTION
Table 2.
Brand substitution status of patients
Atenolol
Citalopram
Enalapril
Metformin
Omeprazole
Ramipril
n ¼ 44 575
n ¼ 18 414
n ¼ 15 752
n ¼ 23 456
n ¼ 67 992
n ¼ 36 814
No switches (%)
35 781 (80)
10 991 (60)
9306 (59)
18 673 (80)
47 017 (69)
32 448 (88)
Brand substitution (%)
5929 (13)
4201 (23)
3543 (22)
3219 (14)
14 639 (22)
3461 (9)
Multiple switches (%)
2865 (6)
3222 (17)
2903 (18)
1564 (7)
6336 (9)
905 (2)
Median switches by multiple
4 (3–5)
4 (3–5)
4 (3–5)
4 (3–5)
4 (3–5)
3 (3–4)
switchers (interquartile range)
The study period was from 1 January 2001 to 28
Statistical
analysis. A
multinomial
generalised
February 2006. Patients were included from their first
estimating equation (GEE) was used to compare the
dispensing post the study start date and followed until
proportion of patients using each drug with no
discontinuation (defined as more than 90 days since
switches, a brand substitution or multiple switches.
the last dispensing), death or study end, whichever
For each drug, a multivariate multinomial logistic
was reached first. Only patients with two or more
regression model was used to determine differences in
dispensings were included, as this is the minimum
brand substitution status and patient age, gender,
number of dispensings required to receive more than
duration of follow-up, number of prescribers and
one product.
number of dispensing pharmacies. No adjustments
were made for multiple comparisons. All analyses
were undertaken using SAS v9.1 (SAS Inc., Cary, NC,
Identification of switches. Switches were identified if
USA).
a patient received different brand or generic products
of the same strength medicine at consecutive dis-
pensings within 60 days. The 60-day interval was
RESULTS
calculated from the data and represents the 90th
percentile for time between prescription refills. If the
Total 160 145 patients met the inclusion criteria. A
manufacturer code was not recorded, it was assumed
quarter of these patients (39 959) received more than
that it was the same as the previous dispensing. Of the
one study drug during follow-up. For all drugs, the
4 000 948 claims identified for the products in this
majority of patients were male and the median age was
study, only 3.0% had no manufacturer code recorded.
over 81 years (see Table 1). The median duration of
follow-up was between 7 and 16 months (Table 1).
Over 80% of patients using each medicine either
had no switches or demonstrated brand substitution
Brand substitution status. Patients who received the
(Table 2). Patients who switched were more likely to
same product throughout follow-up were classified as
have a single brand substitution than multiple switches
non-switchers. Brand substitution was defined if a
(Table 3).
patient had only one switch, or had a total of two
switches involving a switch and then a switch back to
Multiple switchers compared to non-switchers
the original product. Patients with three or more
switches, or who had two switches but received three
Multivariate logistic regression showed small but
different products during follow-up were defined as
statistically
significant
age
differences
between
multiple switchers.
multiple switchers and non-switchers (Table 4). There
Table 3.
Rate ratio (95% CI) for brand substitution status comparisons
Atenolol
Citalopram
Enalapril
Metformin
Omeprazole
Ramipril
No switches versus brand substitution
6.0 (5.9–6.2)
2.6 (2.5–2.7) 2.6 (2.5–2.7)
5.8 (5.6–6.0)
3.2 (3.1–3.3)
9.4 (9.1–9.7)
No switches versus multiple switches
12.5 (12.0–13.0) 3.4 (3.3–3.5) 3.2 (3.1–3.3) 11.9 (11.3–12.6) 7.4 (7.2–7.6) 35.9 (33.6–38.3)
Brand substitution versus multiple
2.1 (2.0–2.2)
1.3 (1.2–1.4) 1.2 (1.2–1.3)
2.1 (1.9–2.2)
2.3 (2.2–2.4)
3.8 (3.6–4.1)
switches
p < 0.0001 for all comparisons.
Copyright # 2008 John Wiley & Sons, Ltd.
Pharmacoepidemiology and Drug Safety, (2008)
DOI: 10.1002/pds
l. m. kalisch ET AL.
were no gender differences between non-switchers
0.0001
0.0001
0.0001
0.0001
0.0001
0.0001
and multiple switchers for patients using atenolol,
acies
<
<
<
<
<
<
p
p
p
p
p
p
citalopram, metformin or omeprazole. When all other
variables in the model were held constant, the odds of
pharm
being a multiple switcher compared to a non-switcher
–1.249)
–1.763)
–1.364)
–1.300)
–1.361)
–1.171)
increased significantly with a 6-month increase in
length of follow-up (Table 4). The odds of being
ispensing
(1.204
(1.630
(1.282
(1.227
(1.316
(1.102
D
6
5
2
3
8
6
a multiple switcher compared to a non-switcher
CI)
increased with increasing number of prescribers and
1.22
1.69
1.32
1.26
1.33
1.13
dispensing pharmacies for all medicines (Table 4).
(95%
in:
Multiple switchers compared to patients with a
01
01
01
01
01
01
brand substitution
increase
0.00
0.00
0.00
0.00
0.00
0.00
<
<
<
<
<
<
For all of the study drugs, multivariate logistic
unit
p
p
p
p
p
p
regression showed no significant differences in the
one
prescribers
92)
52)
12)
15)
88)
86)
age or gender of patients demonstrating brand
a
of
substitution and multiple switchers. The odds of
for
ber
being a multiple switcher compared to having a brand
(1.312–1.3
(1.579–1.7
(1.301–1.4
(1.303–1.4
(1.518–1.5
(1.170–1.2
substitution were significantly greater for patients with
Num
longer follow-up (Table 5). The odds of being a
1.351
1.663
1.355
1.358
1.552
1.227
multiple switcher compared to having a brand
non-switcher
a
substitution increased with increasing number of
to
prescribers and dispensing pharmacies (Table 5).
01
01
01
01
01
01
mpared
DISCUSSION
co
0.00
0.00
0.00
0.00
0.00
0.00
w-up
<
<
<
<
<
<
tcher
p
p
p
p
p
p
For all medicines in this study, at least 80% of patients
cher
follo
56)
43)
33)
47)
63)
73)
did not switch or demonstrated brand substitution.
swit
of
Patients who switched were more likely to have
non-swi
a
nths
a single brand substitution rather than multiple
ltiple
to
mo
.224–1.2
.571–1.6
.292–1.3
.297–1.3
.336–1.3
.313–1.3
switches. Although concerns have been raised that
mu
(1
(1
(1
(1
(1
(1
a
Six
multiple switches occur,5 8,14 results of this study
pared
g
1.240
1.607
1.313
1.322
1.349
1.342
suggest that for the majority of patients (over 80%) the
com
bein
brand substitution policy is working in its current
of
format.
ratio
McManus and colleagues showed that a significant
switcher
02
01
01
33
01
01
number of patients switched to using co-payment
le
Odds
0.00
0.00
0.00
0.04
0.00
0.00
priced products in the 3 months post-introduction of a
¼
<
<
¼
<
¼
brand premium and new generics for ranitidine and
multip
p
p
p
p
p
p
a
ars)
fluoxetine.3 Only a single switch in the time period
11)
19)
19)
13)
15)
00)
(ye
immediately following introduction of the brand
being
–1.0
–1.0
–1.0
–1.0
–1.0
–1.0
premium was considered in their research. Results
ith
Age
of the present study confirm that brand substitution
w
(1.001
(1.010
(1.006
(1.000
(1.008
(0.983
occurs for a wider range of government subsidised
6
4
2
6
2
2
medicines than originally investigated by McManus
1.00
1.01
1.01
1.00
1.01
0.99
sociated
and that substitution is sustained over longer periods
as
of time.
Although the majority of patients received the same
actors
F
le
product throughout follow-up, between 2 and 18% of
4.
patients using each study medicine had multiple
lol
rmin
razo
opram
april
pril
switches. Multivariate logistic regression showed that
able
T
Ateno
Cital
Enal
Metfo
Omep
Rami
multiple switchers attended more pharmacies and had
Copyright # 2008 John Wiley & Sons, Ltd.
Pharmacoepidemiology and Drug Safety, (2008)
DOI: 10.1002/pds
PHARMACEUTICAL BRAND
SUBSTITUTION
Table 5.
Factors associated with being a multiple switcher compared to having a brand substitution
Odds ratio of being a multiple switcher compared to having a brand substitution for a one unit increase in:
(95% CI)
Six months of follow-up
Number of prescribers
Dispensing pharmacies
Atenolol
1.130 (1.114–1.147)
1.063 (1.031–1.097)
1.069 (1.049–1.089)
Citalopram
1.293 (1.267–1.320)
1.200 (1.149–1.254)
1.255 (1.216–1.295)
Enalapril
1.138 (1.119–1.156)
1.103 (1.061–1.146)
1.196 (1.162–1.232)
Metformin
1.139 (1.115–1.163)
1.125 (1.078–1.174)
1.074 (1.045–1.103)
Omeprazole
1.155 (1.143–1.166)
1.151 (1.128–1.174)
1.109 (1.093–1.125)
Ramipril
1.052 (1.027–1.078)
1.075 (1.022–1.130)
1.067 (1.034–1.101)
p < 0.0001 for all comparisons.
more prescribers than other patients; suggesting that
given for inclusion of this principle in future updates
continuity of care between healthcare providers and
of the guiding principles document.
consumers may play a role in multiple switching.
Data for a 5-year period were available for this
Inadequate transfer of information between healthcare
study; however, the median length of follow-up was
providers, consumers and different healthcare settings
less than 16 months. This level of medication
can result in poor quality use of medicines and patient
persistence reflects what occurs in practice and is
harm.15 To address this problem, the Australian
comparable to that seen in other studies.16 19 It is
Pharmaceutical Advisory Council (APAC) has devel-
possible that patients using the medicines in this study
oped guiding principles to achieve continuity in
switched to other treatments or re-initiated therapy
medication management when patients move between
following cessation; however, this was not considered
healthcare settings and providers.15 Although these
for this study.
principles discuss the potential for patient confusion
Although the analysis was limited to DVA treatment
from multiple brand names and the need to ensure
card holders, the medicines studied are equally
that patients understand changes to brands of their
available on the PBS and the co-payments paid by
medicine,15 the issue of multiple brand substitutions is
DVA card holders are the same as PBS concession
not discussed. Healthcare providers should assume
card holders. A comparison of the DVA population
responsibility for maintaining patients on their regular
with the wider Australian population has shown that
brand of medicine wherever possible to minimise the
DVA card holders have slightly more GP visits (rate
likelihood of confusion from multiple substitutions.
ratio 1.17) and hospitalisations (rate ratio 1.21) per
Given the results of this study, consideration should be
year than other Australians. Despite this, DVA card
holders receive only slightly more government
subsidised prescriptions (rate ratio 1.13).20 For this
KEY POINTS
reason, the results are likely to be applicable to other
Australians. There is no evidence to suggest that
Most patients in this study did not have brand
pharmacists are more or less likely to substitute
and generic products substituted.
products for DVA card holders than other Australians.
Patients who had products substituted were more
likely to have a single brand substitution rather
than multiple brand substitutions.
CONCLUSION
Multiple brand substitutions per patient were not
Implementation of the minimum pricing policy and
common, and occurred for less than 18% of
brand substitution was intended to give patients a price
patients using the medicines in this study.
signal and encourage the use of generics,3 it was not
Brand and generic substitution of medicines in
intended to facilitate multiple switches per patient. For
Australia is being implemented primarily as
the drugs included in this study, the brand substitution
intended. It is allowing choice, without leading
policy appears to be having its intended effect for over
to multiple brand substitutions for the majority
80% of patients—that is, allowing choice without
of patients.
facilitating multiple switches. Despite this, some
patients have multiple switches and results of this
Copyright # 2008 John Wiley & Sons, Ltd.
Pharmacoepidemiology and Drug Safety, (2008)
DOI: 10.1002/pds
l. m. kalisch ET AL.
study suggested that continuity of care between
factors associated with poor health outcomes. Age Ageing
different pharmacies and different prescribers may
2005; 34: 626–632.
play a role.
11. Peterson G, Naunton M. Simple measures to assist with drug
therapy can produce large benefits for elderly patients. Aust
Pharmacist 2002; 21(5): 370–373.
12. Department of Veterans’ Affairs. DVA treatment population
ACKNOWLEDGEMENTS
statistics, December 2006. Department of Veterans’ Affairs:
Canberra, 2006. Available from http://www.dva.gov.au/media/
This research was conducted as part of the Veterans’
publicat/Statistics/docs/TpopDec06.pdf [Accessed 29 March
MATES project, funded through the Australian Gov-
2007].
ernment Department of Veterans’ Affairs (DVA) and
13. Australian Government Department of Health and Ageing.
Pharmaceutical Benefits Pricing Authority Annual Report
administered by the University of South Australia.
for the year ended 30 June 2005. Available from http://
www.health.gov.au/internet/wcms/publishing.nsf/Content/
REFERENCES
350C5B2F2D00D2C2CA2570D7001CD22A/$File/04-05%
20PBPA%20Annual%20Report.pdf [Accessed 3rd April 2006].
1. Industry Commission. The Pharmaceutical Industry, Vol. 1. The
14. Ferguson H. GP calls for restrictions on generic substitution.
Report. Australian Government Publishing Service: Melbourne,
Australian Doctor 2006; February 15. Available from:
1996; Report No.: 51.
http://www.australiandoctor.com.au/articles/49/0c03ce49.asp.
2. Australian Government Department of Health and Ageing.
[Accessed 20th February 2008]
Schedule of Pharmaceutical Benefits for Approved Pharmacists
15. Australian Pharmaceutical Advisory Council. Guiding prin-
and Medical Practitioners. Effective from 1 April 2006.
ciples to achieve continuity in medication management.
National Capital Printing: Canberra, 2006.
Australian
Pharmaceutical
Advisory
Council:
Canberra,
3. McManus P, Birkett D, Dudley J, Stevens A. Impact of the
2005.
minimum pricing policy and introduction of brand (generic)
16. Dailey G, Kim M, Lian J. Patient compliance and persistence
substitution into the Pharmaceutical Benefits Scheme in Aus-
with antihyperglycemic drug regimens: evaluation of a Medi-
tralia. Pharmacoepidemiol Drug Saf 2001; 10: 295–300.
caid patient population with type 2 diabetes mellitus. Clin Ther
4. Pharmaceutical Health and Rational Use of Medicines
2001; 23(8): 1311–1320.
(PHARM) Consumer Sub-Committee. Consumer perspectives
17. van Soest E, Siersema P, Dieleman J, Sturkenboom M, Kuipers
on managing multiple medicines. PHARM: Canberra, 2001.
E. Persistence and adherence to proton pump inhibitors in daily
5. Australian Divisions of General Practice Ltd. GPs call for ‘‘one
clinical practice. Aliment Pharmacol Ther 2006; 24: 377–385.
switch per script’’, 2003. Available from http://www.adgp.
18. McManus P, Mant A, Mitchell P, Dudley J. Length of therapy
com.au/client_images/5024.pdf [Accessed 25th October 2004].
with selective serotonin reuptake inhibitors and tricyclic anti-
6. Cain C. Think twice before accepting substitute drugs. The
depressants in Australia. Aust N Z J Psychiatry 2004; 38:
Advertiser, 29 December 2006; Section: Opinion. p. 20.
450–454.
7. Anonymous. Complaints and investigations. Pharmacy Board
19. Bourgault C, Senecal M, Brisson M, Marentette M, Gregoire JP.
of South Australia Newsletter. 2004 August.
Persistence and discontinuation patterns of antihypertensive
8. Deakin G, Aufgang M. Controversies in medicine: wrestling
therapy among newly treated patients: a population-based study.
with brand choices. Aust Doctor 2002; September 13: 42–43.
J Hum Hypertens 2005; 19(8): 607–613.
9. Brooker C. No simple messages on generic substitution. Pharm
20. Australian Institute of Health and Welfare (AIHW). Health care
News 2003; August 21:8.
usage and costs. A comparison of veterans and war widows and
10. Sorensen L, Stokes J, Purdie D, Woodward M, Roberts M.
widowers with the rest of the community. Cat. no. PHE 42.
Medication management at home: medication-related risk
AIHW: Canberra, 2002.
Copyright # 2008 John Wiley & Sons, Ltd.
Pharmacoepidemiology and Drug Safety, (2008)
DOI: 10.1002/pds
Summary of Progress Final Reports
Attachment 6.1 - 6.13
Ethics Id
Date Tabled
Item
Number Study Title
Status
Researcher
Research Institution
Date Submitted
s 22 - Out of scope
E005/009
8/08/2008
6.8
Switching Medicines in the Veteran Population
Final Report
s 47F
University of South Australia
8-Jul-08
s 22 - Out of scope
21/06/2024
1
DVA Ethics Committee – Minutes 15 April 2005
DVA Human Research Ethics Committee
Minutes of the Meeting held on:
Friday 15 April 2005
12th Floor Conference Room - DVA National Office
Present
s 47F
Apologies
s 47F
Agenda Item 1: Opening
The meeting was declared open at 10:30 am by s 47F
and apologies were noted.
Agenda Item 2: Minutes of previous meeting
The Minutes of the meeting held on 10 December 2004 were adopted as a true record of that
meeting with the following comments:
s 22 - Out of scope
Page 1
DVA Ethics Committee – Minutes 15 April 2005
Agenda Item 3: Proposals considered out-of-session
No proposals were considered out-of-session.
s 22 - Out of scope
Page 2
s 22 - Out of scope
s 22 - Out of scope
Page 4
DVA Ethics Committee – Minutes 15 April 2005
s 22 - Out of scope
5.3
Switching medicines in the veteran population and the impact on health outcomes
and costs
(s 47F
, University of South Australia)
The study will look at switching between different priced brands of drugs, and switching
different drugs in the same therapeutic group.
The researcher is seeking access to DVA data.
The Committee unconditionally endorsed the proposal.
s 22 - Out of scope
Page 5
s 22 - Out of scope
s 22 - Out of scope
 DVA HUMAN RESEARCH ETHICS COMMITTEE
DVA HUMAN RESEARCH ETHICS COMMITTEE
Application for consideration of proposed research involving contact
with the veteran community or access to data held by DVA
(External)
This document contains the fol owing parts:
Part A: Study Protocol
Part B: Privacy Considerations
Part C: Agreement
Information Privacy Principles
Version 2000
Current as at 20 February 2001

s 47
3
s 47
4
s 47
5
s 47
6
s 47
7
Back to
Contents
Part B: PRIVACY CONSIDERATIONS
The Commonwealth Privacy Commissioner under section 95 of the Privacy Act 1988
has approved guidelines for the protection of privacy in the conduct of medical
research.
The guidelines apply to a researcher not employed by an Australian Government
agency where that research involves personal information obtained from an
Australian Government agency, the disclosure of which might involve a breach of one
or more Information Privacy Principles (IPPs).
However, as the NHMRC recommends that the guidelines be applied to all research
involving the use of personal information, DVA officers with responsibility for
undertaking studies of the veteran community on behalf of the Department should
also complete this part.
In order that your proposal can be assessed in accordance with the privacy
guidelines, please address each of the following points P1-P11.
s 47
8
s 47
9
Back to
Contents
Part C: AGREEMENT
This agreement relates to a study titled “Switching medicines in the Veteran
Population and the impact on health outcomes and costs”.
I, s 47F
, acknowledge that the information contained in this form is true and
accurate, and I undertake to ensure the security and privacy of the personal
information entrusted to me in accordance with the arrangements described in this
form.
.......................................................…
….. /……/………
Principal Researcher
Date
10
Back to
Privacy Act 1988
Contents
INFORMATION PRIVACY PRINCIPLES
Principle 1 - Manner and purpose of col ection of personal information
1. Personal information shal not be collected by a collector for inclusion in a record or in a generally
available publication unless:
(a) the information is collected for a purpose that is a lawful purpose directly related to a function or
activity of the col ector; and
(b) the collection of the information is necessary for or directly related to that purpose.
2. Personal information shal not be collected by a collector by unlawful or unfair means.
Principle 2 - Solicitation of personal in formation from individual concerned
Where:
(a) a col ector collects personal information for inclusion in a record or in a generally available
publication; and
(b) the information is solicited by the collector from the individual concerned;
the collector shall take such steps (if any) as are, in the circumstances, reasonable to ensure that,
before the information is col ected or, if that is not practicable, as soon as practicable after the
information is collected, the individual concerned is generally aware of:
(c) the purpose for which the information is being col ected;
(d) if the col ection of the information is authorised or required by or under law - the fact that the
collection of the information is so authorised or required; and
(e) any person to whom, or any body or agency to which, it is the col ector's usual practice to
disclose personal information of the kind so collected, and (if known by the collector) any
person to whom, or any body or agency to which, it is the usual practice of that first mentioned
person, body or agency to pass on that information.
Principle 3 - Solicitation of personal information general y
Where:
(a) a col ector collects personal information for inclusion in a record or in a generally available
publication; and
(b) the information is solicited by the collector;
the collector shall take such steps (if any) as are, in the circumstances, reasonable to
ensure that,
having regard to the purpose for which the information is collected:
(c) the information collected is relevant to that purpose and is up to date and complete; and
(d) the collection of the information does not intrude to an unreasonable extent upon the personal
affairs of the individual concerned.
Principle 4 - Storage and security of personal information
A record-keeper who has possession or control of a record that contains personal information shall
ensure:
(a) that the record is protected, by such security safeguards as it is reasonable in the circumstances to
take, against loss, against unauthorised access, use, modification or disclosure, and against other
misuse, and
(b) that if it is necessary for the record to be given to a person in connection with the provision of a
service to the record-keeper, everything reasonably within the power of the record-keeper is done
to prevent unauthorised use or disclosure of information contained in the record
Principle 5 - Information relating to records kept by record-keeper
1. A record-keeper who has possession or control of records that contain personal information shall,
subject to clause 2 of this Principle, take such steps as are, in the circumstances, reasonable to
enable any person to ascertain:
(a) whether the record-keeper has possession or control of any records that contain personal
information; and
(b) if the record-keeper has possession or control of a record that contains such information:
(i)
the nature of that information;
(ii)
the main purposes for which that information is used; and
(iii)
the steps that the person should take if the person wishes to obtain access to the
record.
2. A record-keeper is not required under clause I of this Principle to give a person information if the
record-keeper is required or authorised to refuse to give that information to the person under the
applicable provisions of any law of the Commonwealth that provides for access by persons to
documents.
3. A record-keeper shal maintain a record setting out:
(a) the nature of the records of personal information kept by or on behalf of the record-keeper;
(b) the purpose for which each type of record is kept;
(c) the classes of individuals about whom records are kept;
(d) the period for which each type of record is kept;
11
(e) the persons who are entitled to have access to personal information contained in the records
and the conditions under which they are entitled to have that access; and
(f) the steps that should be taken by persons wishing to obtain access to that information.
4. A record-keeper shal :
(a) make the record maintained under clause 3 of this Principle available for inspection by members
of the public; and
(b) give the Commissioner, in the month of June in each year, a copy of the record so maintained.
Principle 6 - Access to records containing personal information
Where a record-keeper has possession or control of a record that contains personal information, the
individual concerned shal be entitled to have access to that record, except to the extent that the
record-keeper is required or authorised to refuse to provide the individual with access to that record
under the applicable provisions of any law of the Commonwealth that provides for access by persons to
documents.
Principle 7 - Alteration of records containing personal information
1. A record-keeper who has possession or control of a record that contains personal information shall
take such steps (if any), by way of making appropriate corrections, deletions and additions as are,
in the circumstances, reasonable to ensure that the record:
(a) is accurate; and
(b) is having regard to the purpose for which the information was col ected or is to be used and to
any purpose that is directly related to that purpose, relevant, up to date, complete and not
misleading.
2. The obligation imposed on a record-keeper by clause I is subject to any applicable limitation in a
law of the Commonwealth that provides a right to require the correction or amendment of
documents.
3. Where:
(a) the record-keeper of a record containing personal information is not wil ing to amend that
record, by making a correction deletion or addition, in accordance with a request by the
individual concerned; and
(b) no decision or recommendation to the effect that the record should be amended whol y or partly
in accordance with that request has been made under the applicable provisions of a law of the
Commonwealth;
the record-keeper shal , if so requested by the individual concerned, take such steps (if any) as are
reasonable in the circumstances to attach to the record any statement provided by that individual of the
correction, deletion or addition sought.
Principle 8 - Record-keeper to check accuracy etc of personal information before use
A record-keeper who has possession or control of a record that contains personal information shall not
use that information without taking such steps (if any) as are, in the circumstances, reasonable to
ensure that, having regard to the purpose for which the information is proposed to be used, the
information is accurate, up to date and complete
Principle 9 - Personal information to be used only for relevant purposes
A record-keeper who has possession or control of a record that contains personal information shall not
use the information except for a purpose for which the information is relevant.
Principle 10 - Limits on use of personal information
1. A record-keeper who has possession or control of a record that contains personal information that
was obtained for a particular purpose shall not use the information for any other purpose unless:
(a) the individual concerned has consented to use of the information for that other purpose;
(b) the record-keeper believes on reasonable grounds that use of the information for that other
purpose is necessary to prevent or lessen a serious and imminent threat to the life or health of
the individual concerned or another person;
(c) use of the information for that other purpose is required or authorised by or under law;
(d) use of the information for that other purpose is reasonably necessary for enforcement of the
criminal law or of a law imposing a pecuniary penalty, or for the protection of the public
revenue; or
(e) the purpose for which the information is used is directly related to the purpose for which the
information was obtained.
Where personal information is used for enforcement of the criminal law or of a law imposing a pecuniary
penalty, or for the protection of the public revenue, the record-keeper shall include in the record
containing that information a note of that use.
Principle 11 - Limits on disclosure of personal information
1. A record-keeper who has possession or control of a record that contains personal information shall
not disclose the information to a person, body or agency (other than the individual concerned)
unless:
(a) the individual concerned is reasonably likely to have been aware, or made aware under
Principle 2, that information of that find is usually passed to that person, body or agency;
12
(b) the individual concerned has consented to the disclosure;
(c) the record-keeper believes on reasonable grounds that the disclosure is necessary to prevent
or lessen a serious and imminent threat to the life or health of the individual concerned or of
another person;
(d) the disclosure is required or authorised by or under law; or
(e) the disclosure is reasonably necessary for the enforcement of the criminal law or of a law
imposing a pecuniary penalty, or for the protection of the public revenue.
2. Where personal information is disclosed for the purposes of enforcement of the criminal law or of a
law imposing a pecuniary penalty, or for the purpose of the protection of the public revenue, the
record-keeper shal include in the record containing that information a note of the disclosure.
3. A person, body or agency to whom personal information is disclosed under clause I of this Principle
shall not use or disclose the information for a purpose other than the purpose for which the
information was given to the person, body or agency.
PRIVACY COMMISSIONER, GPO Box 5218, SYDNEY, NSW, 2001
Privacy Hotline 1800 023 985 Telephone (02) 9284 9600 TTY 1800 620 241 Fax (02) 281 9666
13
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
s 47
DVA Human Research Ethics Committee
Meeting to be held
Friday 15 April 2005
10:30am – 12:30pm
12th Floor Conference Room
DVA National Office
Lovett Tower
Woden ACT
Agenda Items
1.
Opening
2.
Minutes of previous meeting
3.
Proposals considered out-of-session
4.
Previously considered proposals
5.
New proposals
6.
Progress reports
7.
Other business
8.
Closure
DVA Ethics Committee – 15 April 2005 Agenda
Agenda Item 1: Opening
The meeting is scheduled to open at 10:30am. Please note that this meeting will be held in
the 12th floor conference room.
Agenda Item 2: Minutes of previous meeting
Minutes of the meeting held on 11 February 2005 is at
Attachment 2.1.
• Matters arising from the Minutes.
Agenda Item 3: Proposals considered out-of-session
No proposals were considered out-of session.
s 22 - Out of scope
2
s 22 - Out of scope
s 22 - Out of scope
s 22 - Out of scope
5.3
Switching medicines in the veteran population and the impact on health outcomes
and costs
(s 47F
University of South Australia)
The study will look at switching between different priced brands of drugs, and switching
different drugs in the same therapeutic group.
The researcher is seeking access to DVA data.
The documentation can be found at
Attachment 5.3.
s 22 - Out of scope
5
s 22 - Out of scope
Attachment 4.1
2nd March 2005
Mr Robert s 47F
Director of Medication Management
Department of Veterans’ Affairs
PO Box 21
Woden ACT 2606
Dear Bob
Re: DVA Veterans’ MATES Ethics Application
Please find attached an additional ethics application, together with a copy of
the original application as referenced in the attached, for consideration at the
next DVA Human Research Ethics Commit ee meeting.
If you would like any further information or additional details that would assist
in the commit ee’s consideration of this matter, please do not hesitate to
contact either myself or s 47F
.
Yours sincerely
s 47F
s 47F
: DVA
Veterans’ MATES project
University of South Australia
s 47F
s 47F
s 47F
Co
NATION
ntact:
Diane
s
AL OFFICE
47F
s 47F
Telephone: s 47F
School of Pharmacy and Biomedical Sciences
Facsimile: (02) 6289 4776
s 47F
University of South Australia
E-mail:
diane.
@dva.gov.au
ADELAIDE 5000
Prescriber Intervention and Feedback Program – Veterans’ Medicines Advice and
Therapeutics Education Services
Change in protocol: attaching a barcode to prescriber and veteran response forms
Dear s 47F
Thank you for submitting the above change in protocol for consideration by the DVA Human
Research Ethics Committee. The Committee considered it at its meeting on 15 April 2005.
The Committee had no ethical or privacy concerns with the change and endorsed the new
protocol.
I would like to remind you that, as part of their monitoring role, the Committee must be:
• advised, in writing and before implementation, should protocols change in the future.
• provided with progress reports and/or final reports.
The Committee looks forward to receiving your progress/final report in due course.
If you would like to discuss this matter further, please contact me in the first instance on
s 47F
or via the Committee’s e-mail address (xxxxxx.xxxxxxxxx@xxx.xxx.xx).
Yours sincerely
Diane s 47F
Ethics Committee Coordinator
19 April 2005
s 22 - Out of scope
s 22 - Out of scope
5.3 Switching medicines in the Veteran Population and the impact on health outcomes and
costs
I have no privacy concerns regarding this proposal as the principal researcher will access only
de-identified data and the study does not involve direct contact with individual veterans.
Item 27. It is proposed that only de-identified prescriptions claims data will be used. The
research proposal is part of the MATES project and the researcher is proposing to access de-
identified data from that project for use in this research proposal.
Item 28. The study does not involve contact with veterans. The research will be mostly
statistical tests and analysis of de-identified data sourced from the data already supplied to QPRC
for purposes of the MATES project.
I have no concerns with regard to the proposed use and storage protocols outlined under Part B.




















